Our research efforts have focused on protein-protein interactions in signaling pathways involved in cell fate regulations such as proliferation, differentiation, and cell death. Using an interdisciplinary strategy combining theoretical and experimental approaches, we wish to crack the molecular basis underlying protein-protein interactions in these important biological processes, and design novel inhibitors to modulate the characterized protein-protein interactions.
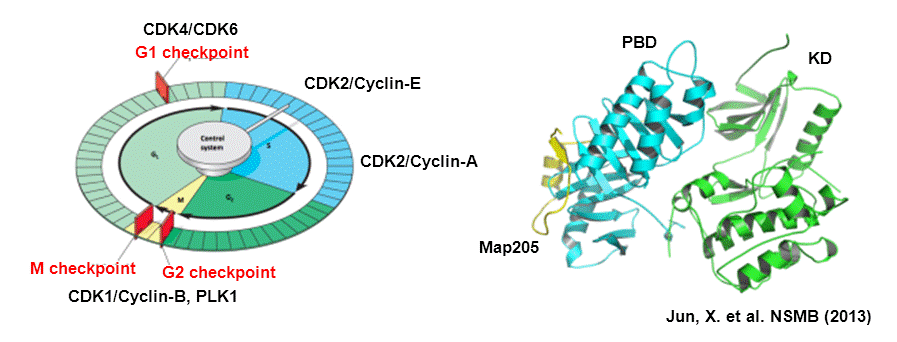
1. Protein kinases and Cell cycle regulations
Protein kinases orchestrate the complex events that drive the cell cycle, their activity is tightly regulated in a tempo-spatial manner. Dysfuntions of protein kinases are closely associated with diverse diseases such as cancer, inflammatory diseases, and neurological disorders. Protein kinases are therefore important targets for drug development. Among more than five hundred protein kinases, CDKs and Plks are the master regulators of the multiple stages of cell cycle, which promote cell cycle progression or monitor the checkpoint of cell cycle progression. We focus on the regulating mechanisms underlying their physiological functions through protein-protein interactions on the molecular level, and also study the small molecule modulators targeting protein-protein interactions.
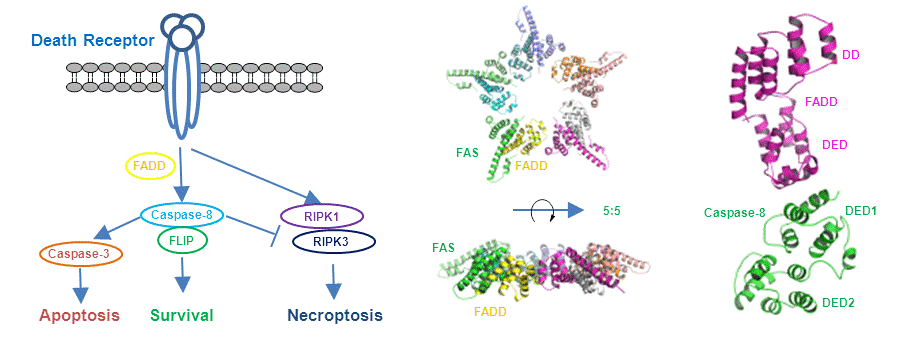
2. Caspase-8, cell death and cell survival
Caspase-8 plays crucial roles in the cell death and survival processes through complex regulating network, which activates apoptosis via death-inducing signaling complex (DISC), and blocks necroptosis by inhibiting the necrosome. Formation of DISC is mediated by homotypic interaction between the cytosolic death domain (DD) of the death receptor Fas and the DD of the adaptor protein FADD, and by homotypic interaction between the death-effector domain (DED) of FADD and the DEDs of caspase-8. The homotypic interaction between FADD and caspase-8 are also important in the inhibition of necroptosis. Despite extensive studies of DISC, the molecular basis for these homotypic interactions remain largely unknown. We focus on the structural study of caspase-8 and the complexes of caspase-8 with its partners.