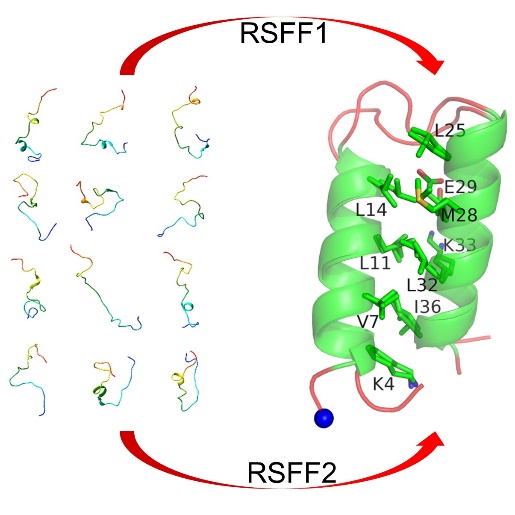
Wu group published article on J. Phy. Chem. B. about Folding Simulations of an α-Helical Hairpin Motif αtα with Residue-Specific Force Fields.
-helical hairpin (t) is a structure motif composed of two interacting helices connected by a turn or a short loop. It is an important model for protein folding studies, filling the gap between isolated -helix and larger all- domains. There are lots of experiments aiming to understand the folding mechanism. While the conflicting results confuse us, it calls for the further research to unveil the folding mechanism of t. In this work, we present, for the first time, the successful folding simulations of t. In spite of RSFF1 and RSFF2 used, the simulations gave very similar predicted structures of this t peptide, which is in good agreement with its NMR structure.
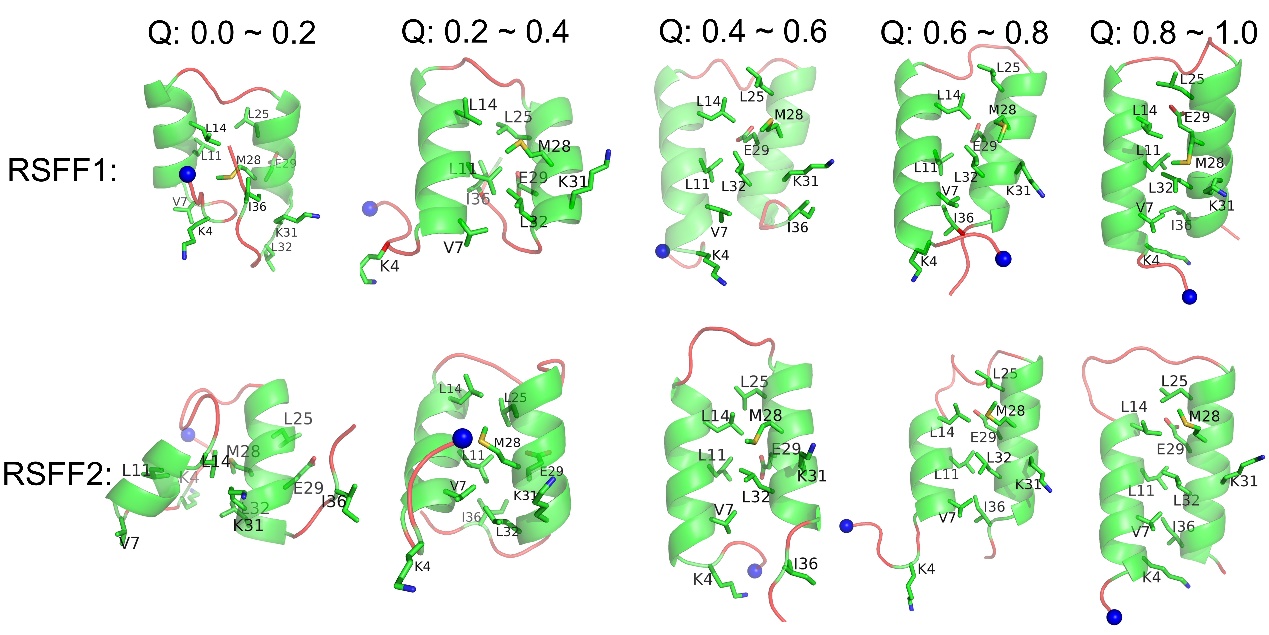
Combining the folding free energy landscapes and analyses of structures sampled in five different ranges of the fraction of native contacts (Q), a folding mechanism of ata is proposed. The most stable sites of Q9-E15 in helix-1 and E24-A30 in helix-2 close to the loop region act as the folding initiation sites. The formation of inter-helix side-chain contacts also initiates near the loop region, but some residues in the central parts of the two helices also form contacts quite early. The two terminus fold at a final stage, and the loop region keep flexible during the whole folding process. This mechanism is similar to the “zipping out” pathway of a-hairpin folding.
post-doctoral Juan Zeng is the first author, This article was published in the “The Journal of Physical Chemistry B” (Zeng, J.; Jiang, F.; Wu, Y.-D., Folding Simulations of an α-Helical Hairpin Motif αtα with Residue-Specific Force Fields. The Journal of Physical Chemistry B, DOI: 10.1021/acs.jpcb.5b09027).
Linking: http://pubs.acs.org/doi/abs/10.1021/acs.jpcb.5b09027