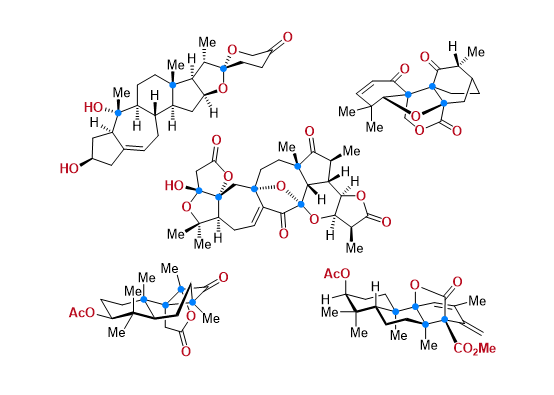
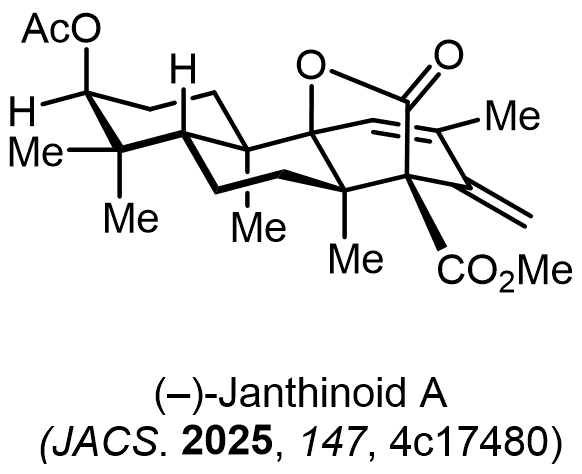
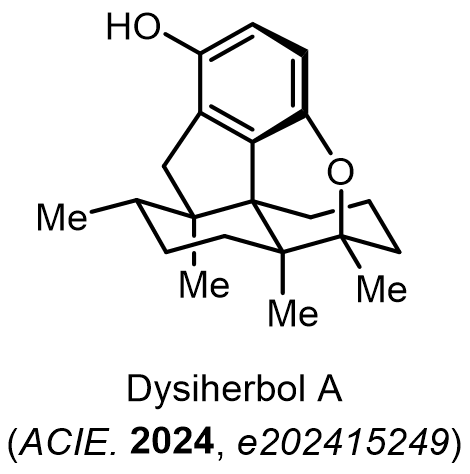
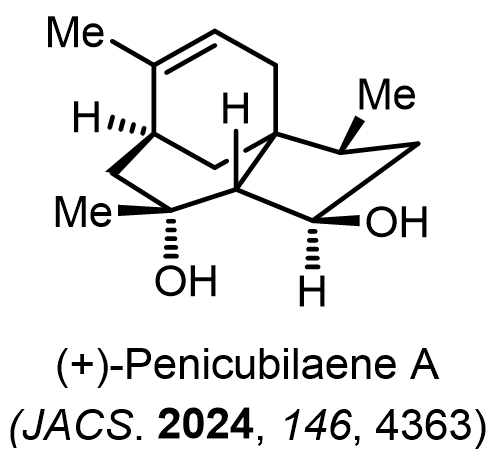
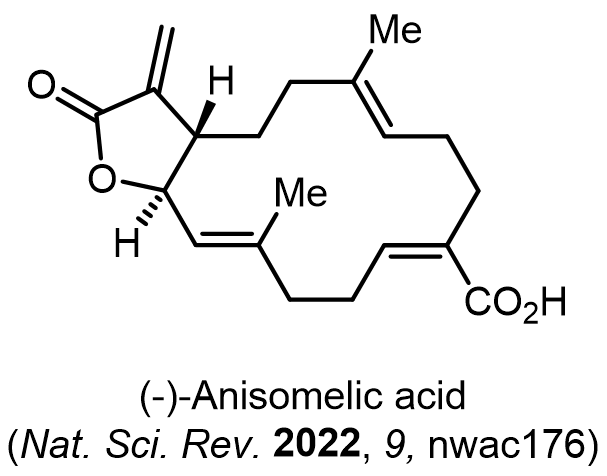
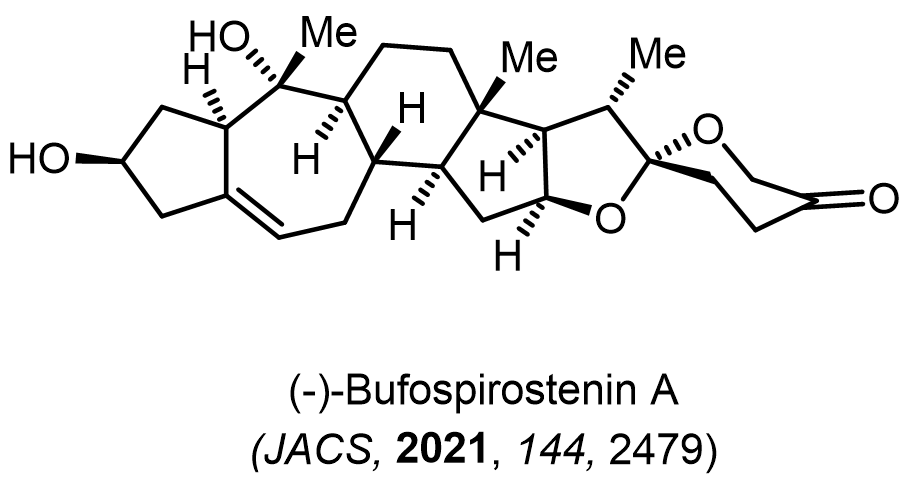
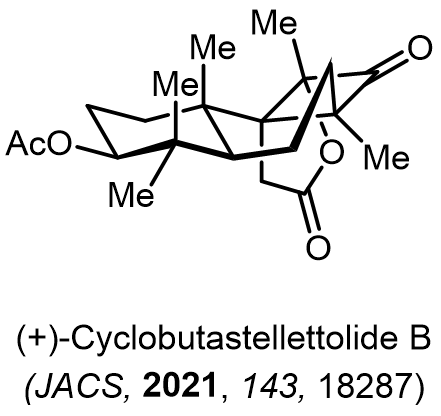
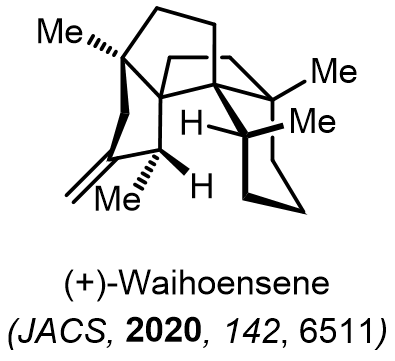
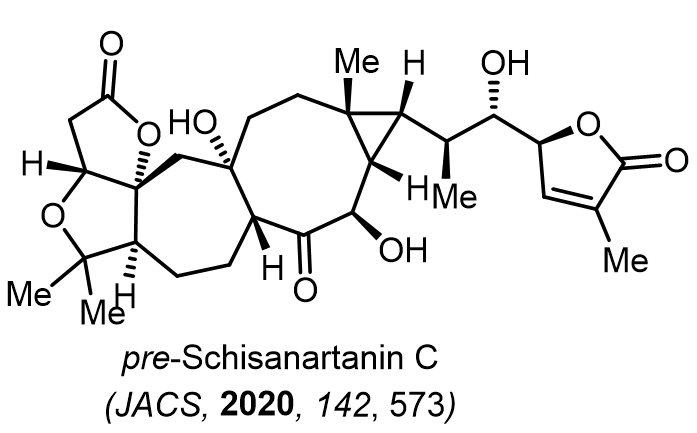
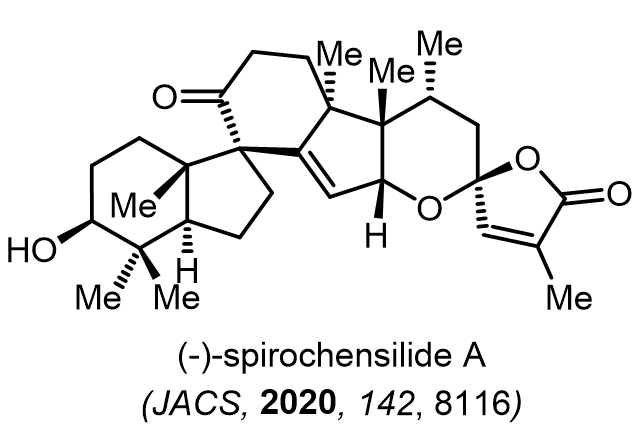
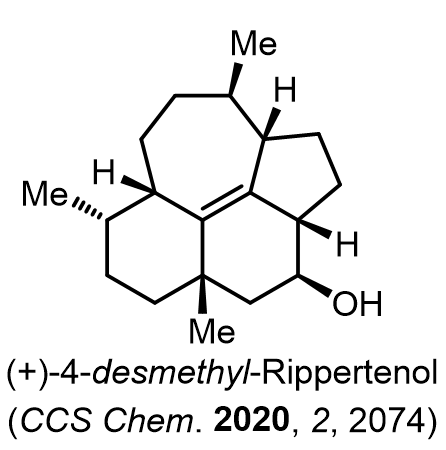
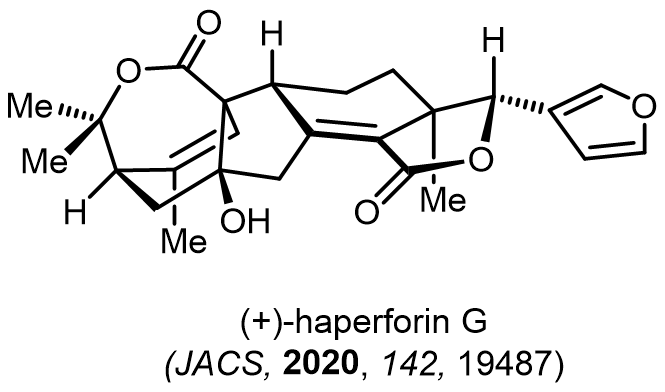
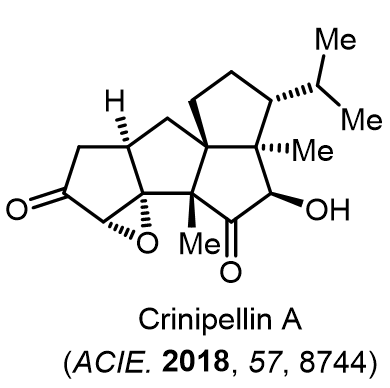
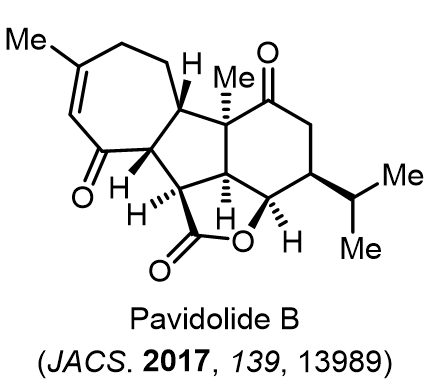
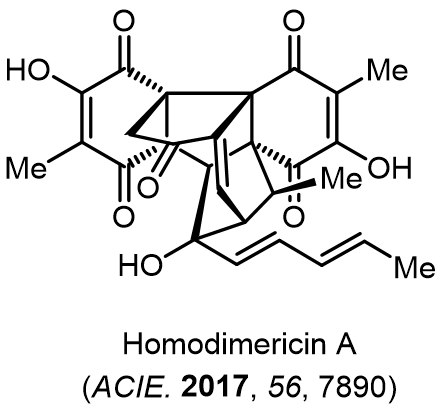
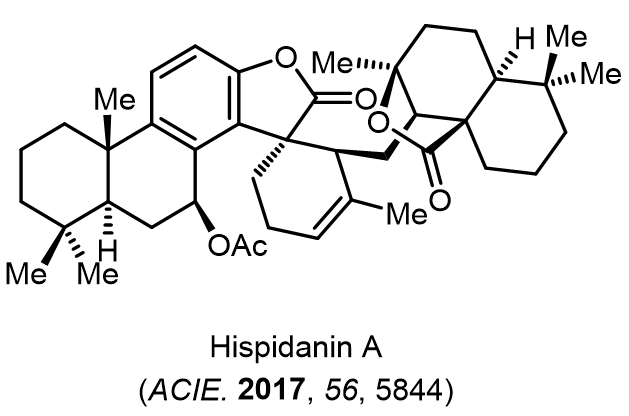
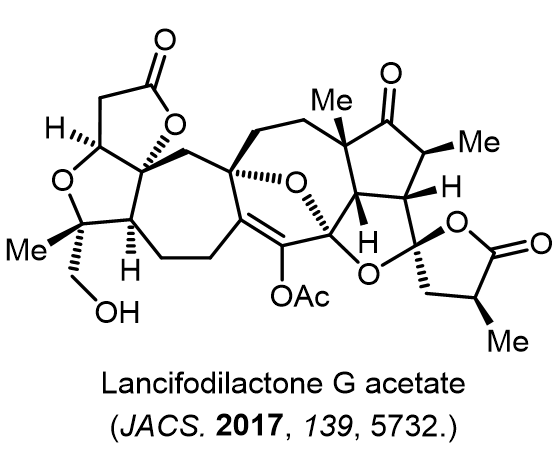
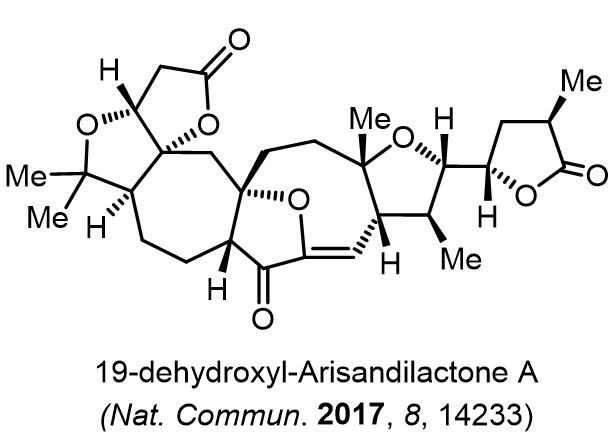
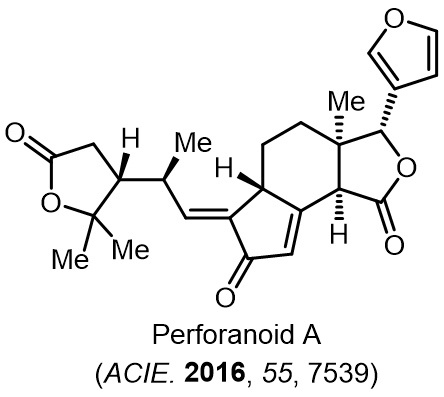
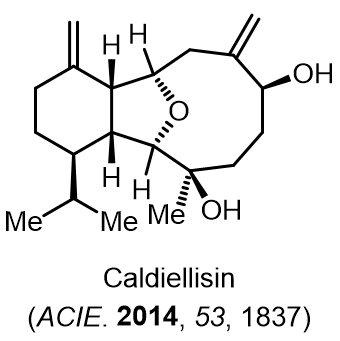
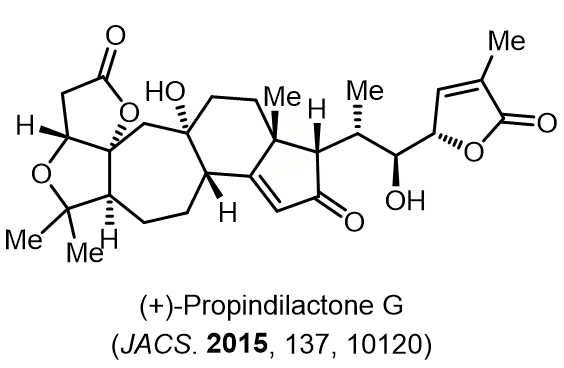
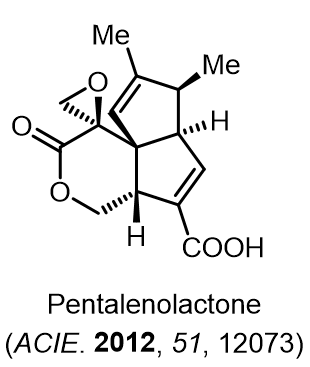
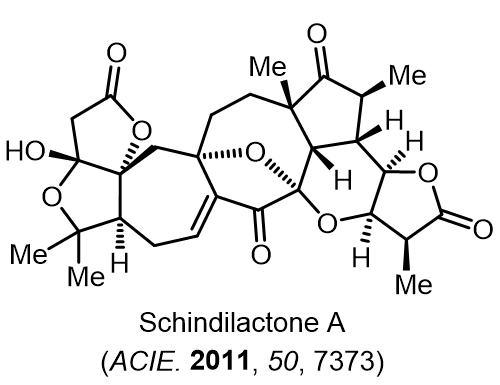
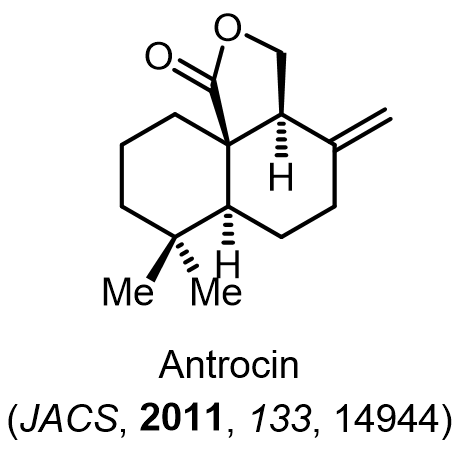
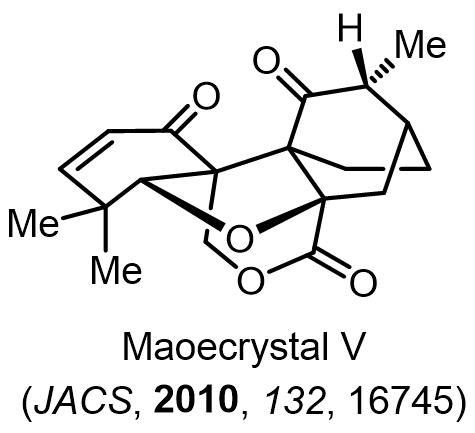
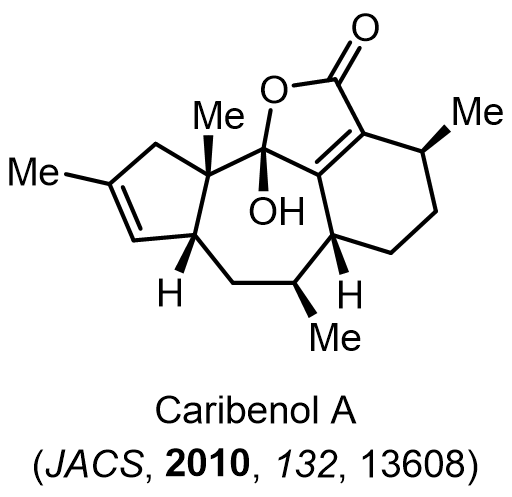
Total Synthesis of Bioactive Natrual Products
Our group has made significant strides in synthesizing complex and biologically active natural products and their derivatives through innovative strategies and the discovery of new chemical methodologies. Documented in 247 publications, our research merges expertise in target-oriented synthesis with an increasing emphasis on biological and drug discovery applications.
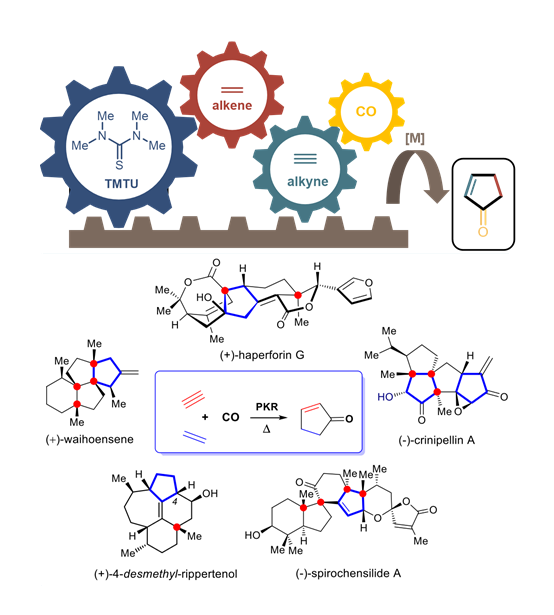
Exploring the Thiourea Ligand in Pauson-Khand Reaction: A Powerful Tool for Natural Product Synthesis
The Pauson-Khand (PK) reaction is one of the most efficient methods for constructing cyclopentenones, which are key frameworks in many natural products. Our group has also developed synthetic strategies to access biologically active complex natural products while searching for methods to enhance the efficiency of Pauson-Khand (PK) reactions. We identified Co2(CO)8/tetramethyl thiourea (TMTU) as tetramethyl thiourea (TMTU) as a new catalyst that facilitates PK reactions under balloon pressure of CO at 70 °C.
Medicinal Chemistry of Natural Products
In addition to our total synthesis research, our group has continued and accelerated collaborative programs with drug discovery companies under the auspices of the State Key Laboratory of Chemical Oncogenomics at Peking University Shenzhen Graduate School. We have designed a new strategy for developing “non-immunosuppressive” cyclosporine as an anti-inflammatory drug. In this initiative, we...