-
J. Am. Chem. Soc. 2025, 146 4731–4735
Fu Tang, Zhong-Chao Zhang*,Zhi-Lin Song, Yuan-He Li, Zi-Hao Zhou, Jia-Jun Chen and Zhen Yang*
The asymmetric total synthesis of janthinoid A has been accomplished for the first time in 14 steps without using a protecting group. The trans-decalin subunit and the rigid oxabicyclo[3.2.1]octane motif were constructed via an epoxide-initiated cationic π-cyclization reaction and a Fe(ClO4)3-mediated oxidative cascade cyclization reaction, respectively -
Angew. Chem. Int. Ed. 2025, 64, e202415249
Bing-Yan Liu+, Zi-Chun Zhang+, Zhi-Lin Song, Hong-Yi Yuan, Yuan-He Li, Zhong-Chao Zhang,* Zhen Yang*
The Norrish–Yang reaction, as a typical example, demonstrates the inherent ability of photochemical reaction to facilitate formation of sterically congested C-C bonds, efficiently crafting intricate ring structure in complex organic molecules. Herein we report for the first time a unified synthesis using quinone-based acid-promoted Norrish–Yang photocyclization for the stereoselective constru... -
Tetrahedron Lett. 2024, 145, 155161.
Fu Tang, Zhongchao Zhang*, Zhen Yang*
Poly-substituted 2-hydroxycyclobutanone motifs are core motifs in many biologically important natural products, we recently developed Norrish–Yang photocyclization/1,2-methyl migration as a synthetic strategy for regio/stereoselectively constructing them and profiled the scope and limitations of the proposed strategy in this work -
Org. Lett. 2024, 26, 8217–8221.
Yi-Ke Zhou, Zhen-Yu Zhang, Hao-Yuan Liu, Yuan-He Li, Zhong-Chao Zhang, Jia-Hua Chen*, Zhen Yang*
A Norrish-Yang photocyclization reaction has been applied to regio- and stereoselective construction of the ABCDE pentacyclic motif of natural product phainanoids. The observed substrate conformation control implicates this powerful reaction could be applied to the construction of structurally diverse natural product scaffolds -
Chem. Eur. J. 2024. 30(17), e202304371
Yuan-He Li,* Jia-Hua Chen, and Zhen Yang*
The Diels–Alder reaction stands as one of the most pivotal transformations in organic chemistry. Its efficiency, marked by the formation of two carbon-carbon bonds and up to four new stereocenters in a single step, underscores its versatility and indispensability in synthesizing natural products and pharmaceuticals. The most significant stereoselectivity feature is the “endo rule”. While thi... -
Org. Lett. 2024, 26(26), 5403−5408
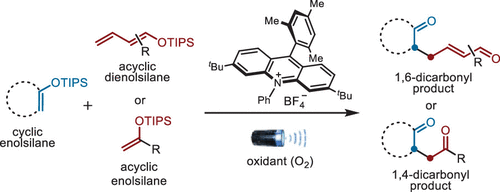
Xinyu Tan, Zhilin Song, Xinting Liang, Zhenbao Wang, Hongyi Yuan, Zhongchao Zhang,*and Zhen Yang*
A photoredox-based oxidative heterocoupling of enolsilanes to the corresponding 1,4- and 1,6-dicarbonyl compounds was developed by using Mes-Acr+BF4– as the photocatalyst, and oxygen was used as the oxidant. This newly developed chemistry adheres to the principles of atom economy, step economy, and redox economy, making it a concise and efficient method. -
J. Org. Chem. 2023, 88(15), 10539−10554
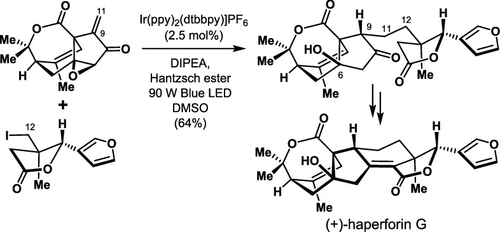
Zhenyu Zhang, Wei Zhang, Jun−Chen Tang, Jin−Teng Che, Zhongchao Zhang, Jia−Hua Chen,*and Zhen Yang*
(+)-Haperforin G was synthesizedin 20 steps from commerciallyavailable starting materials. A Co-catalyzed intramolecular Pauson-Khandreaction was used for stereoselective construction of cyclopentanonebearing an all-carbon quaternary stereogenic center at the bridge-headposition. Light-initiated photocatalysis was used for convergent andasymmetric cross-coupling of the unstabilized C(sp(3)) rad... -
Org. Lett. 2024, 26(15), 2960–2964
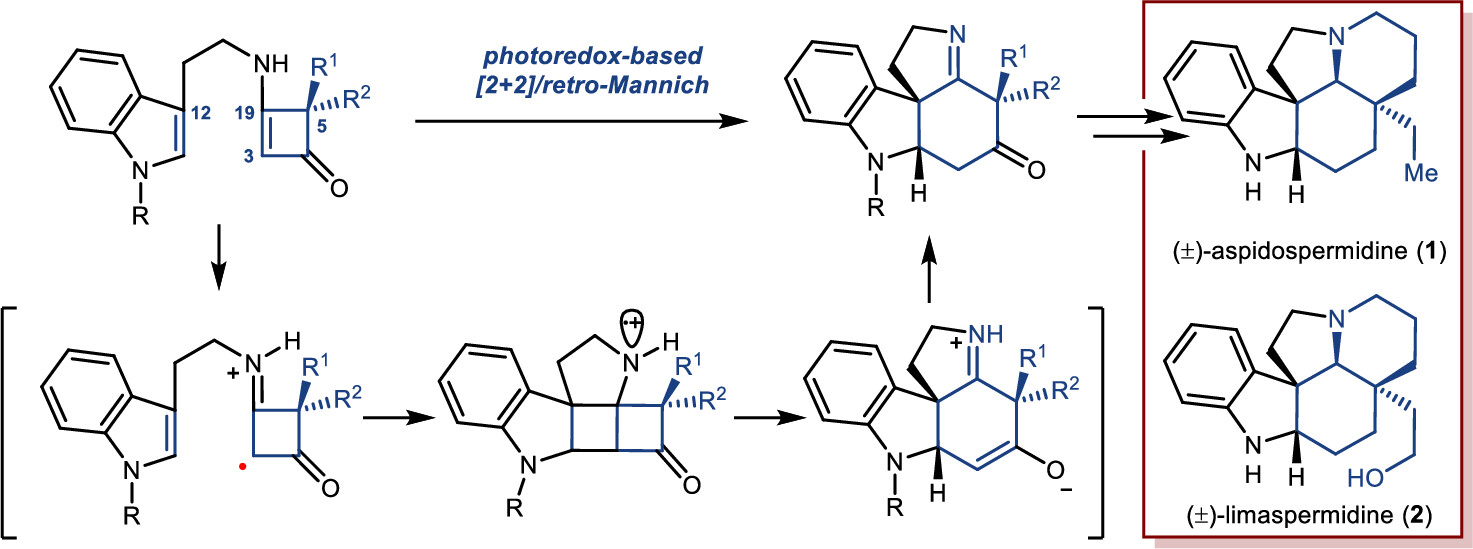
Jianyu Long, Rudong Liu, Xinpeng Mu, Zhilin Song, Zhongchao Zhang,* and Zhen Yang*
A novel strategy for the synthesis of Aspidosperma alkaloids has been achieved via a photoredox-initiated [2+2]/retro-Mannich reaction of tryptamine-substituted enaminones as a key step. The developed chemistry has been applied to the construction of the core tetracycle of Aspidosperma alkaloids (±)-aspidospermidine and (±)-limaspermidine -
J. Am. Chem. Soc.2024, 146(7), 4363–4368
Zheyuan Wang, Zhilin Song, Jun Huang,* and Zhen Yang*
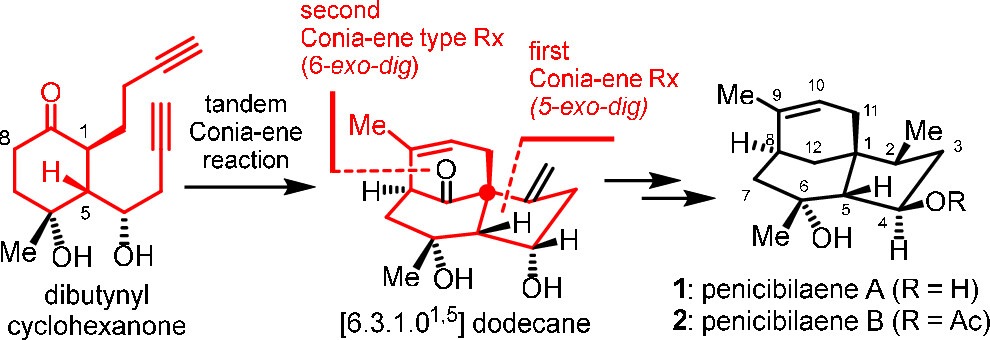
The total syntheses of penicibilaenes A and B are described. The key step is thetBuOK/DMSO-mediated tandem 5-exo–digConia-ene type reaction and 6-exo–digConia-ene type reaction to install the tricyclic [6.3.1.01,5] dodecane core of penicibilaenes from dibutynyl cyclohexanone in a single step, together with a sequence of copper-mediated conjugate addition and Crabtree’s hydrogenation to forge... -
Chem Asian J. 2023, 18, e20230062
Hao-Yuan Liu, Zhen-Yu Zhang, Yi-Ke Zhou, Jia-Hua Chen, Zhen Yang,* and Yuan-He Li*
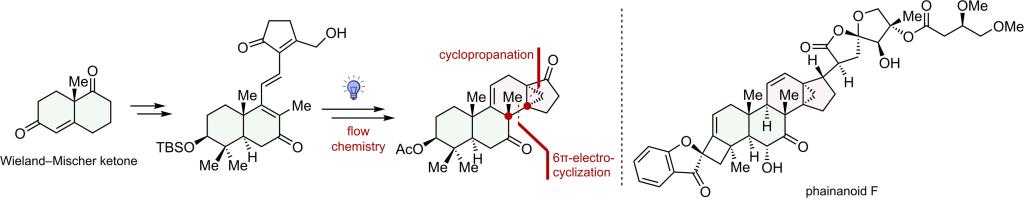
In this paper, we report an efficient strategy for synthesizing the DEFGH rings of phainanoid F. The key to the construction of the 13,30-cyclodammarane skeleton of the molecule was a photo-induced 6π-electrocyclization and a homoallylic elimination. Notably, this is a rare example of using electrocyclization reaction to simultaneously construct two vicinal quaternary carbons in total synthesi...