Yuan-He Li, Su-Lei Zhang, Yong Lu, Bo Xiao, Tian-Yu Sun, Qian-Qian Xu, Jia-Hua Chen,* Zhen Yang*
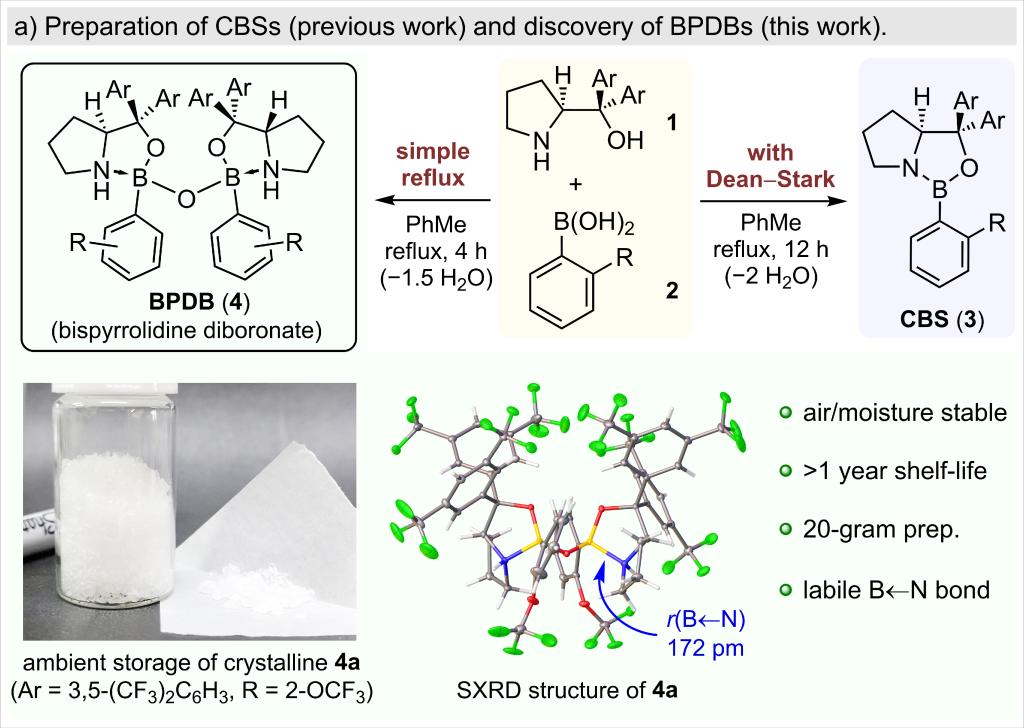
A highly enantioselective catalytic system for exo-Diels–Alder reactions was developed based on the newly discovered bispyrrolidine diboronates (BPDBs). When activated various Lewis or Brønsted acids, BPDBs could catalyze highly stereoselective asymmetric exo-Diels–Alder reactions of monocarbonyl-based dienophiles. When 1,2-dicarbonyl-based dienophiles are used, the catalyst can sterically distinguish between the two binding sites, which leads to highly regioselective asymmetric Diels–Alder reactions. BPDBs can be prepared as crystalline solids on a large scale and are stable under ambient conditions. Single-crystal X-ray analysis of the structure for acid-activated BPDB indicated that activation involves breakage of a labile N→B bond.