Junkai Fu, Hongjuan Shen, Yuanyuan Chang, Prof. Dr. Chuangchuang Li, Dr. Jianxian Gong and Prof. Dr. Zhen Yang*
Chem. Eur. J. 2014, 20, 12881
• Highlighted in Org. Chem. Highlights, May, 11th, 2015.
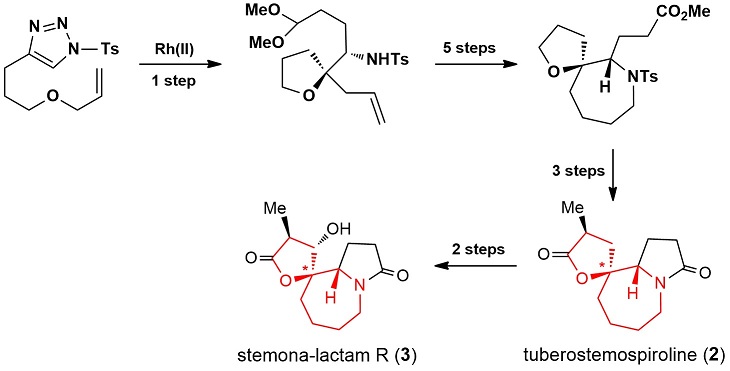
A 4-substituted-1-tosyl-1,2,3-triazole-based stereoselective synthesis of structurally diverse oxaspirocycles is reported. The synthesis involves Rh-catalyzed loss of nitrogen from 4-substituted-1-tosyl-1,2,3-triazoles, Grignard reaction, and a ring-closing metathesis reaction as key steps. By employing readily available and stable 4-substituted-1-tosyl-1,2,3-triazoles as surrogates of diazo compounds and nitrogen sources, two types of oxaspirocycles were obtained. The latter compounds, which contain adjacent nitrogen stereocenters, could serve as the core structures of many natural products. This chemistry has been successfully applied to the total syntheses of (±)-tuberostemospiroline and (±)-stemona-lactam R.