J. Liu, H. Wang, H. Zhang, X. Wu, H. Zhang, Y. Deng, Z. Yang*, A. Lei*
Chem. Eur. J. 2009, 15, 4437.
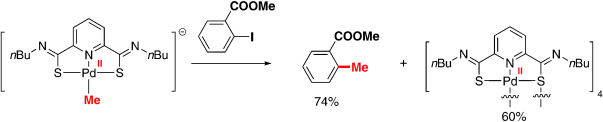
PdIIate complex: A novel alkylated pincer thioimido–Pd complex generated from a catalyst precursor and basic organometallic reagents (RM) was observed by in situ IR,1H NMR, and 13C NMR spectroscopies for the first time and proved to be the active catalyst in stoichiometric and catalytic reactions of aryl iodides with RM (see scheme). The catalyst, as an electron-rich PdII species, promoted the Negishi coupling of aryl iodides and alkylzinc reagents with high efficiency, even at low temperatures (0 or −20 °C).