Wei-bin Zhang, Wen-bin Shao, Fu-zhuo Li, Dr. Jian-xian Gong*, Prof. Dr. Zhen Yang*
Chem. Asian J. 2015, 10, 1874.
• Highlighted in Org. Chem. Highlights 2016, August 22.
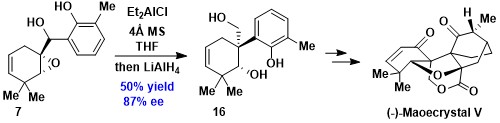
The asymmetric total synthesis of (−)-maoecrystal V, a novel cytotoxic pentacyclic ent-kaurane diterpene, has been accomplished. Key steps of the current strategy involve an early-stage semipinacol rearrangement reaction for the construction of the C10 quaternary stereocenter, a rhodium-catalyzed intramolecular O−H insertion reaction, and a sequential Wessely oxidative dearomatization/intramolecular Diels–Alder reaction to forge the pentacyclic framework of maoecrystal V.