Nan Zheng, Yuan-Yuan Chang, Li-Jie Zhang, Jian-Xian Gong,* and Zhen Yang*
Chem. Asian J. 2016, 11, 371
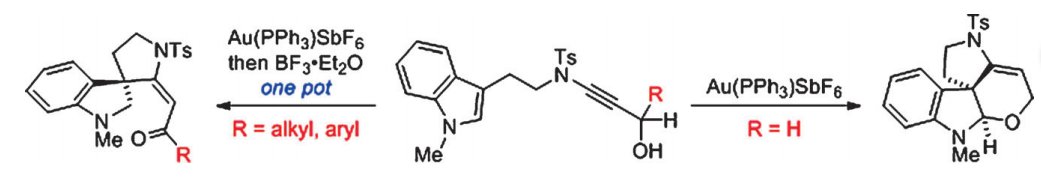
A gold-catalyzed intramolecular tandem cyclization of indole-ynamide affords tetracyclic spirocyclic pyrrolidinoindoline bearing an all-carbon quaternary stereocentre in a single step; however, when the reaction was carried out in the presence of BF3⋅Et2O, the corresponding tricyclic spirocyclic pyrrolidinoindoline-based enones are produced through a key 1,5-hydride shift. The developed chemistry provides a diastereoselective and straightforward entry to structurally diverse polycylic pyrrolidinoindolines from indole-ynamides in one-pot reactions under mild conditions.