Fu-Zhuo Li, Shuang Li, Peng-Peng Zhang, Zhi-Hui Huang, Wei-Bin Zhang,* Jianxian Gong* and Zhen Yang*
Chem. Commun., 2016,52, 12426-12429
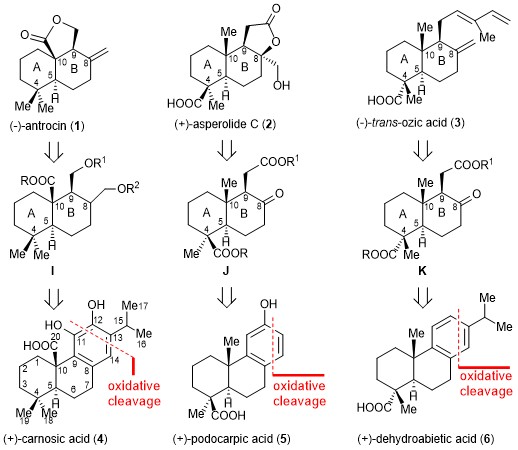
Ozonolysis of aromatic abietane (+)-carnosic acid (4) is used to create an important intermediate in an enantiomerically pure form, resulting in a simple, concise, readily scalable, and asymmetric synthesis of (−)-antrocin (1). This strategy not only provides an efficient approach to (−)-antrocin (1) synthesis but can also be readily adopted for the syntheses of optically pure (+)-asperolide C (2) and (−)-trans-ozic acid (3) from the naturally abundant aromatic abietanes (+)-podocarpic acid (5) and (+)-dehydroabietic acid (6). The strategy presented here is an example of the use of naturally occurring aromatic abietanes as a chiral pool and offers an account of the asymmetric synthesis of terpenoids.
Inside back cover
