Hai-Xin Yu, Nan Zheng, Chi-Tai Yeh, Chien-Ming Lee, Qi Zhang, Wen-Lv Zheng, Qing Chang, Yuan-He Li, Yu-Jun Li, Gui-Zhen Wu∗, Jun-Min Quan∗, Lin-Qi Zhang∗, Yew-Min Tzeng∗ and Zhen Yang∗
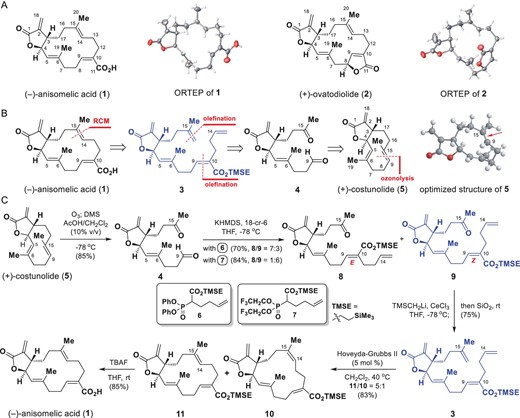
(−)-Anisomelic acid, isolated from Anisomeles indica (L.) Kuntze (Labiatae) leaves, is a macrocyclic cembranolide with a trans-fused α-methylene-γ-lactone motif. Anisomelic acid effectively inhibits SARS-CoV-2 replication and viral-induced cytopathic effects with an EC50 of 1.1 and 4.3 μM, respectively. Challenge studies of SARS-CoV-2-infected K18-hACE2 mice showed that oral administration of anisomelic acid and subcutaneous dosing of remdesivir can both reduce the viral titers in the lung tissue at the same level. To facilitate drug discovery, we used a semisynthetic approach to shorten the project timelines. The enantioselective semisynthesis of anisomelic acid from the naturally enriched and commercially available starting material (+)-costunolide was achieved in five steps with a 27% overall yield. The developed chemistry provides opportunities for developing anisomelic-acid-based novel ligands for selectively targeting proteins involved in viral infections.