Guozong Yue, Yun Zhang, Lichao Fang, Chuang-chuang Li, Tuoping Luo,* and Zhen Yang*
Angew. Chem. Int. Ed. 2014, 53, 1837
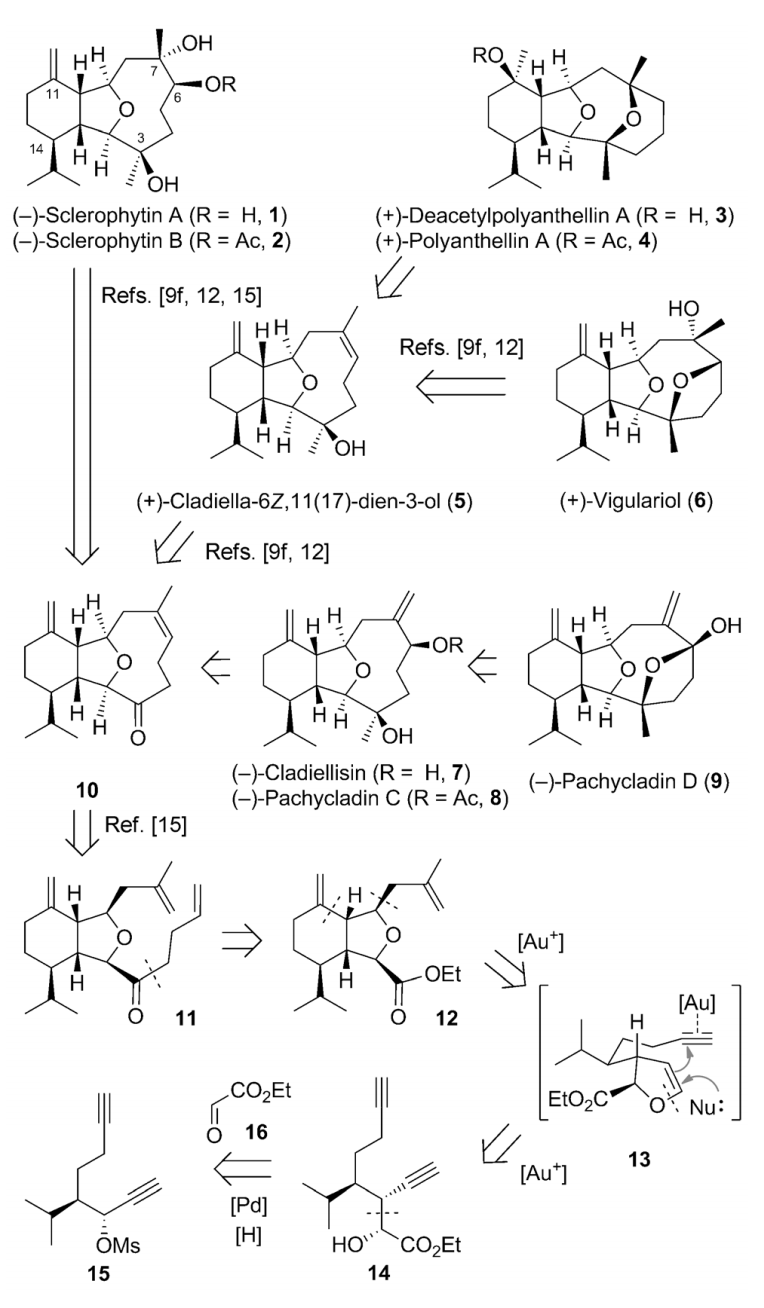
The cladiellin family of natural products, which includes molecules with various biological activities, continues to invite new synthetic studies. A gold-catalyzed tandem reaction of 1,7-diynes to construct the 6-5-bicyclic ring systems that are present in a number of natural products was developed. This reaction was applied as the key step to realize the formal and total syntheses of nine members of the cladiellin family in an enantio- and diastereoselective manner. This modular and efficient approach could also be used for the construction of other cladiellins, as well as their analogues, for follow-up studies.