Dr. Guang Lin, Dr. Le Chang, Dr. Yongxiang Liu, Dr. Zheng Xiang, Prof. Dr. Jiahua Chen,* andProf. Dr. Zhen Yang,*
Chem. Asian J., 2013, 8, 700.
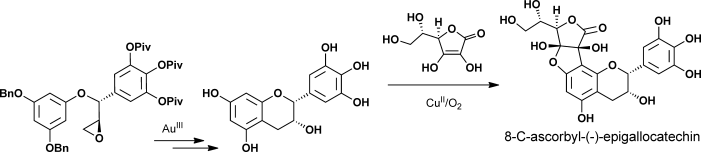
Reading the tea leaves: The enatioselective total syntheses of 8-C-ascorbyl-(−)-epigallocatechin was accomplished by CuII-mediated oxidative coupling of ascorbic acid and (−)-epigallocatechin as a key step. Also, the asymmetric total syntheses of tea-leaf extracts (+)-gallocatechin and (−)-epigallocatechin were achieved by Au-catalyzed intramolecular cycliarylation of the precursor epoxide and Sharpless dihydroxylation.