News&Event
125.Asymmetric Total Synthesis of Lancifodilactone G Acetate. 1. Diastereoselective Synthesis of CDEFGH Ring System
Tian-Wen Sun, Dong-Dong Liu, Kuang-Yu Wang, Bing-Qi Tong, Jia-Xin Xie, Yan-Long Jiang, Yong Li, Bo Zhang, Yi-Fan Liu, Yuan-Xian Wang, Jia-Jun Zhang, Jia-Hua Chen*, Zhen Yang*
J. Org. Chem., 2018, 83 (13), 6893–6906
The stereoselective construction of the CDEFGH ring system of lancifodilactone G is described. The key steps in this synthesis are (i) ring-closing metathesis for formation of the oxa-bridged eight-membered ring; (ii) an intramolecular Pauson–Khand reaction for construction of the sterically congested F ring; and (iii) sequential cross-metathesis, hydrogenation, and lactonization reactions for installation of the anomerically stabilized bis-spiro ketal fragment of lancifodilactone G.
124.Asymmetric Total Synthesis of Lancifodilactone G Acetate. 2. Final Phase and Completion of the Total Synthesis
Kuang-Yu Wang, Dong-Dong Liu, Tian-Wen Sun, Yong Lu, Su-Lei Zhang, Yuan-He Li, Yi-Xin Han, Hao-Yuan Liu, Cheng Peng, Qin-Yang Wang, Jia-Hua Chen*, Zhen Yang*
J. Org. Chem.2018.83 (13), 6907–6923
The asymmetric total synthesis of lancifodilactone G acetate was accomplished in 28 steps. The key steps in this synthesis include (i) an asymmetric Diels–Alder reaction for formation of the scaffold of the BC ring; (ii) an intramolecular ring-closing metathesis reaction for the formation of the trisubstituted cyclooctene using a Hoveyda–Grubbs II catalyst; (iii) an intramolecular Pauson–Khand reaction for construction of the sterically congested F ring; (iv) sequential cross-metathesis, hydrogenation, and lactonization reactions for installation of the anomerically stabilized bis-spiro ketal fragment of lancifodilactone G; and (v) a Dieckmann-type condensation reaction for installation of the A ring. The strategy and chemistry developed for the total synthesis will be useful in the synthesis of other natural products and complex molecules.
123.Stereoselective Total Synthesis of (±)-5-epi-Cyanthiwigin I via an Intramolecular Pauson–Khand Reaction as the Key Step
Yuanyuan Chang, Linlin Shi, Jun Huang, Lili Shi, Zichun Zhang, Hong-Dong Hao*, Jianxian Gong*, and Zhen Yang*
A convenient approach to the construction of the 5–6–7 tricarbocyclic fused core structure of cyanthiwigins via a Co-mediated Pauson–Khand reaction as a key step has been developed. The cyathane core intermediate obtained by this strategy was used in the concise synthesis of (±)-5-epi-cyanthiwigin I. The developed chemistry paves the way for the total synthesis of structurally diverse cyanthiwigins.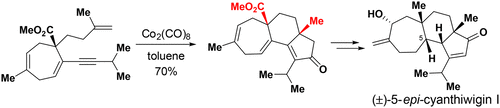
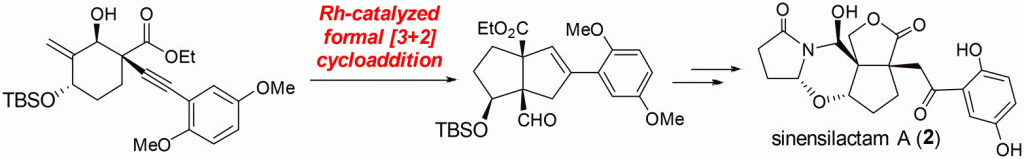
122.Total Synthesis of Sinensilactam A
Wenbin Shao, Jun Huang, Kai Guo, Jianxian Gong*, and Zhen Yang*
The total synthesis of naturally occurring (±)-sinensilactam A was achieved in 18 steps. The key steps of this work are a rhodium-catalyzed [3 + 2] cycloaddition for construction of the two all-carbon vicinal quaternary centers and a convergent and tandem condensation of the in situ generated N-acyliminium intermediate with aldehyde 20. This enabled implementation of a unified strategy for stereoselective formation of the tetracyclic hemiaminal core of sinensilactam A in a later stage. The total syntheses of applanatumol F and C8-epi-applanatumol D are also achieved using this strategy.
Prof. Yang wins Asian Core Program Lectureship Award
Prof. Yang attended the 12th International Conference on Cutting-Edge Organic Chemistry in Aisa (ICCEOCA-12), and wins Asian Core Program Lectureship Award.



