News&Event
119.Diastereoselective Synthesis of Diquinanes and Triquinanes Bearing Vicinal Quaternary Carbon Stereocenters from Acyclic Allene-based Precursors via a Cascade Reaction
Shuang Li, Pengpeng Zhang, Yuanhe Li, Shumin Lu, Jianxian Gong*, and Zhen Yang*
A cascade benzenethiol-mediated intramolecular [3 + 2] cycloaddition reaction between an allene and an α,β-unsaturated aldehyde or ester is developed for the diastereoselective synthesis of [3.3.0] bicyclic system bearing two quaternary atoms at their bridgehead positions. Notably, these structurally complex systems can be found in a wide range of natural products.

118.Asymmetric total synthesis of (−)-perforanoid A
Chao, Lv ; Qian, Tu ; Jianxian, Gong*; Xiaojiang, Hao*; Zhen, Yang*
Asymmetric total synthesis of (−)-perforanoid A, a novel limonoid isolated from the leaves ofHarrisonia perforata, has been achieved. The key features of our total synthesis include the Rh-catalyzed intramolecular Pauson–Khand reaction of an allene and an alkene, the Pd-catalyzed lactonization of an allylic alcohol with a vinyl ether, and the enantioselective alkenylation of 3-furaldehyde.
117.A rhodium-catalyzed tandem reaction of N-sulfonyl triazoles with indoles: access to indole-substituted indanones
An efficient strategy for the synthesis of structurally diverse indole-substituted indanones via a rhodium(II)-catalyzed tandem reaction of N-sulfonyltriazoles with indoles was developed. The reaction involves rhodium(II)-catalyzed denitrogenation of the N-sulfonyltriazoles to form an oxonium ylide, followed by nucleophilic addition of the indoles and subsequent skeletal rearrangement. This strategy provides straightforward access to indanone frameworks bearing quaternary carbon centers.

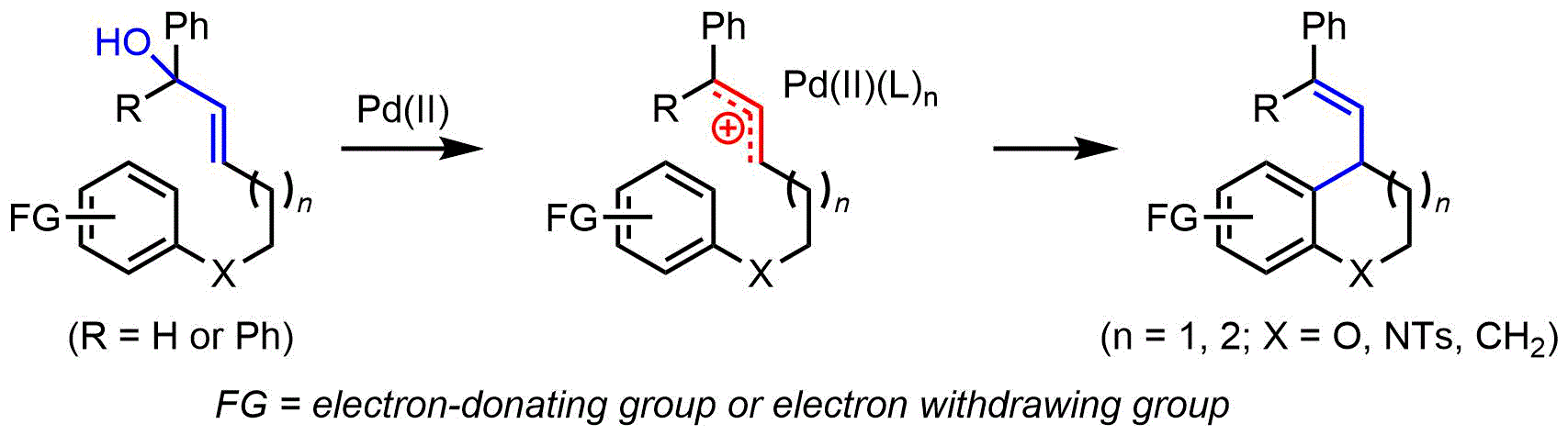
116.Synthesis of Substituted Benzoxacycles via a Pd(II)-Catalyzed Intramolecular Arylation Reaction of Allylic Alcohols
Jingjie Li, Ceheng Tan, Xinpeng Mu, Jianxian Gong*, Zhen Yang*
Herein a Pd-catalyzed intramolecular allylation reaction of unprotected allylic alcohols was developed, and the reaction proceeded through a Pd(II)-mediated allylic carbocation species formation, followed by a Friedel-Crafts type annulation to afford functionalized chromanes.
115. Biomimetically inspired asymmetric total synthesis of (+)-19-dehydroxyl arisandilactone A
Yi-Xin Han, Yan-Long Jiang, Yong Li, Hai-Xin Yu, Bing-Qi Tong, Zhe Niu, Shi-Jie Zhou, Song Liu, Yu Lan*, Jia-Hua Chen*, Zhen Yang*
Complex natural products are a proven and rich source of disease-modulating drugs and of efficient tools for the study of chemical biology and drug discovery. The architectures of complex natural products are generally considered to represent significant barriers to efficient chemical synthesis. Here we describe a concise and efficient asymmetric synthesis of 19-dehydroxyl arisandilactone A—which belongs to a family of architecturally unique, highly oxygenated nortriterpenoids isolated from the medicinal plant Schisandra arisanensis. This synthesis takes place by means of a homo-Michael reaction, a tandem retro-Michael/Michael reaction, and Cu-catalysed intramolecular cyclopropanation as key steps. The proposed mechanisms for the homo-Michael and tandem retro-Michael/Michael reactions are supported by density functional theory (DFT) calculation. The developed chemistry may find application for the synthesis of its other family members of Schisandraceae nortriterpenoids.

