News&Event
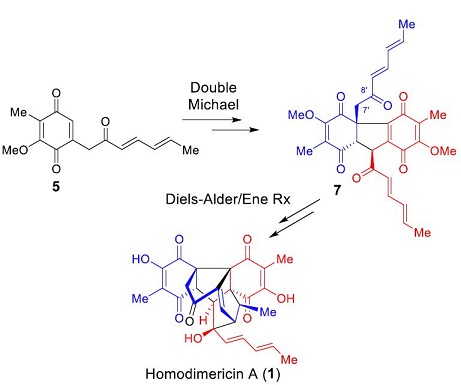
111. Bioinspired Total Synthesis of Homodimericin A
Jun Huang, Yueqing Gu, Kai Guo, Lei Zhu, Yu Lan*, Jianxian Gong* and Zhen Yang*
Angew. Chem. Int. Ed. 2017, 56, 7890
• Highlighted in Synfacts, 2017, 13, 906.
Homodimericin A is a remarkable fungal metabolite, and a highly oxygenated and racemic unsaturated polyketide. It poses a significant synthetic challenge due to its sterically demanding central cage-like core bearing eight contiguous stereogenic centers (including three contiguous all-carbon quaternary stereocenters), and several carbonyl functionalities. Based on its proposed biogenetic synthesis, we designed a bioinspired total synthesis of homodimericin A that proceeds in seven steps, featuring a double Michael reaction, an intramolecular Diels-Alder reaction, and an ene reaction.
110.Bioinspired Asymmetric Synthesis of Hispidanin A
Fuzhuo Li, Qian Tu, Sijia Chen, Lei Zhu, Yu Lan,* Jianxian Gong,* and Zhen Yang*
Angew.Chem. Int. Ed. 2017, 56,5844
• Selected as Hot Paper in Natural Products
• Highlighted in Synfacts, 2017, 13, 569.
The first enantiospecific synthesis of hispidanin A (4), a dimeric diterpenoid from the rhizomes of Isodon hispida, was achieved with a longest linear sequence of 12 steps in 6.5 % overall yield. A key component is the use of the abundant and naturally occurring diterpenoids (+)-sclareolide and (+)-sclareol as starting materials, which enables the gram-scale preparation of the key intermediates totarane (1) and s-trans-12E,14-labdadien-20,8β-olide (2). Subsequently a thermal or an erbium-catalyzed intermolecular Diels–Alder reaction of totarane (1) with labdadienolide (2) provide convergent and rapid access to the natural product hispidanin A (4). The synthetic studies have offered significant impetus for the efficient construction of these architecturally complex natural products.

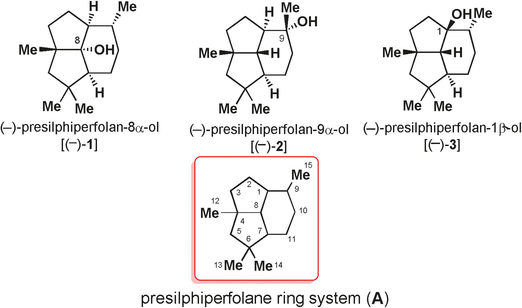
109. A Concise Synthesis of Presilphiperfolane Core through a Tandem TMTU–Co-Catalyzed Pauson–Khand Reaction and a 6π Electrocyclization Reaction
Zichun Zhang, Yuanhe Li, Dandan Zhao, Yingdong He, Dr. Jianxian Gong*, Prof. Dr. Zhen Yang*
The synthesis of strained polycyclic systems from readily available precursors with a minimum number of steps and with regio- and stereochemical control constitutes an important synthetic challenge. Herein, we report a tandem reaction comprising Co–TMTU (tetramethyl thiourea)-catalyzed Pauson–Khand (PK) and 6π-electrocyclization reactions for the formation of the highly strained core of presilphiperfolanols. The developed chemistry has been applied to the total syntheses of 4-epi-presilphiperfolan-8-ol and 7-epi-presilphiperfolan-1-ol.
Congratulations on Prof. Yang and Prof. Gong on winning the second class national natural science award for 2016
The national science and technology reward was held in Beijing Great Hall of the People on January 9, 2017. In the conference, the second class national natural science award for 2016 was announced. Professor Yang and Prof. Gong, together with Prof. Tang and Prof. Chen from Tsinghhua University and Peking University respectively won the second class prize of the national science and technology reward for 2016 by their research in “the total synthesis of natural products with significant biological activities”. This research belonged to the field of organic synthetic chemistry and natural product total synthesis. The research teamrealized the first total synthesis of more than 20 natural products.
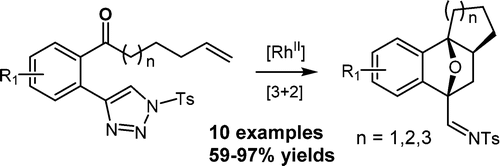
108.Stereoselective Synthesis of Oxabicyclo[2.2.1]heptenes via a Tandem Dirhodium(II)-Catalyzed Triazole Denitrogenation and [3 + 2] Cycloaddition
Hao Yuan, Jianxian Gong*, and Zhen Yang*
Org. Lett., 2016, 18 (21), 5500–5503
A novel synthetic strategy for the diastereoselective synthesis of structurally diverse oxabicyclo[2.2.1]heptenes has been developed, featuring a tandem reaction combining a Rh-catalyzed triazole denitrogenation and a novel type of [3 + 2] cycloaddition reaction. This tandem reaction was thought to proceed via a five-membered oxonium ylide intermediate, which was formed by the intramolecular nucleophilic attack of the carbonyl group on the α-imino metallocarbene followed by an inter- or intramolecular [3 + 2] dipolar cycloaddition with a range of alkynes and alkenes.