News&Event
48.Construction of All-Carbon Quaternary Center by R2AlCl−Mediated Ring-Opening Reaction of Oxacycles
C. Che, L. Z. Liu, J. X. Gong, Y. F. Yang, G. X.Wang, J. M. Quan* and Z. Yang*
An unexpected R2AlCl-mediated ring-opening reaction of oxacycles for the formation of all-carbon quaternary centers was discovered, and a possible mechanism is proposed. The developed chemistry provides a concise approach to synthesize structural diverse of dolastane-type compounds.

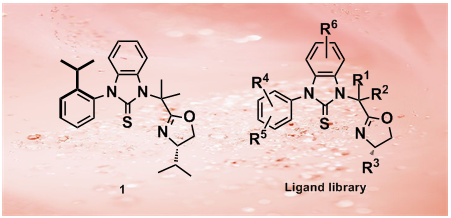
47.A Thiourea-Oxazoline Library with Axial Chirality: Ligand Synthesis and Studies of the Palladium-Catalyzed Enantioselective Bis(methoxycarbonylation) of Terminal Olefins
Y. X. Gao, L. Chang, H. Shi, B. Liang, K. Wongkhan, D. Chaiyaveij, A.S. Batsanov, T. B. Marder*, C. C. Li*, Z.n Yang*, Y. Huang*
Adv, Synth Catal. 2010, 352, 1955
We report herein the synthesis of novel chiral S,N-heterobidentate thiourea-oxazoline ligands and their application to palladium-catalyzed enantioselective bis(methoxycarbonylation)s of terminal olefins under mild conditions. Copper salts were found to play multiple roles in this reaction. Substituted 2-phenylsuccinates were obtained in >90% yield and up to 84% ee under optimized conditions.
46.Development of New Stereodiverse Diaminocyclitols as Inhibitors of Influenza Virus Neuraminidase
Y. Cui, Z. D. Jiao, J. X. Gong, Q. Yu, X. F. Zheng, J. M. Quan*, M. Luo* and Z. Yang*
A concise and modular approach to synthesize a new type of cyclopentene-based diaminocyclitol library from d-serine and l-serine has been developed, and key steps in this synthesis are an aza-Claisen rearrangement, a ring-closing metathesis, and a Baylis−Hillman reaction. The developed chemistry may offer a unique way to investigate the neuraminidase (NA) mutation by systematically mapping the changes within its binding sites.

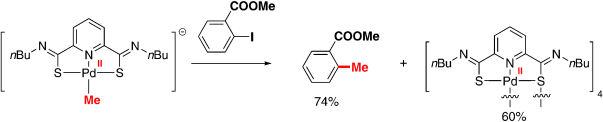
45. Identification of a Highly Efficient Alkylated Pincer Thioimido-Palladium(II) Complex as the Active Catalyst in Negishi Coupling
J. Liu, H. Wang, H. Zhang, X. Wu, H. Zhang, Y. Deng, Z. Yang*, A. Lei*
PdIIate complex: A novel alkylated pincer thioimido–Pd complex generated from a catalyst precursor and basic organometallic reagents (RM) was observed by in situ IR,1H NMR, and 13C NMR spectroscopies for the first time and proved to be the active catalyst in stoichiometric and catalytic reactions of aryl iodides with RM (see scheme). The catalyst, as an electron-rich PdII species, promoted the Negishi coupling of aryl iodides and alkylzinc reagents with high efficiency, even at low temperatures (0 or −20 °C).
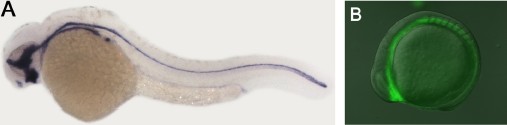
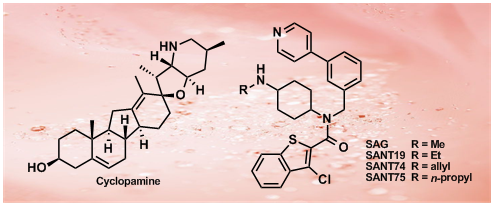
44.Converse Conformational Control of Smoothened Activity by Structurally Related Small Molecules
H. B. Yang, J. Xiang N. D. Wang, Y. Zhao, J. Hyman, S. Li, J. Jiang, J. K. Chen, Z. Yang*, S. Lin*
J Biol Chem. 2009, 284, 20876.
The seven-pass transmembrane protein Smoothened (Smo) is an essential component of the Hedgehog (Hh) signaling pathway that is critically involved in normal animal development as well as pathological malignancies. In studying Hh-related biological processes, it would be highly desirable if Smo activity could be instantly switched between activation and inhibition. Using Gli1-dependent GFP transgenic zebrafish and in vitro biochemical assays, we identified and characterized two potent Smo inhibitors, SANT74 and 75 (Smoothened antagonist 74 and 75), by screening a small molecule library designed based on the scaffold of Smo agonist SAG. These compounds are structural analogs of SAG with the methyl group substituted by a propyl or allyl group in SANTs. We show that SANTs and SAG exert opposite effects on Smo activity by regulating protein conformation. Our study represents the first demonstration of conformational regulation of Smo by small molecule analogs, and the combinational use of these Smo modulators in a temporal controlled fashion should be useful for studying Hh biology.