News&Event
6.Synthesis of Novel Palladacycles and Their Application in Heck and Suzuki Reactions under Aerobic Conditions
Z. C. Xiong, N. D. Wang, M. J. Dai, A. Li, J. H. Chen*, Z. Yang*
Design and synthesis of a novel family of furancarbothioamide-based palladacycles are reported herein. These palladacycles are thermally stable, not sensitive to air or moisture, and are applied effectively in the Heck reaction of aryl halides with terminal olefins and in the Suzuki reaction of aryl halides with arylboronic acids. These reactions were performed under aerobic conditions, leading to turnover numbers (TONs) up to 1 × 105.
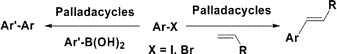
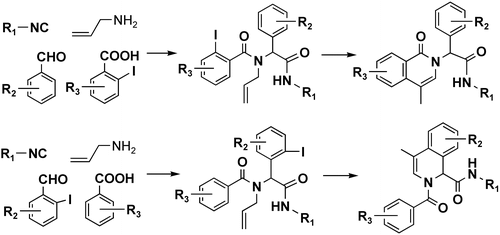
5.Concise Syntheisis of Isoquinoline via the Ugi and Heck Reactions
Z. Xiang, T. P. Luo, K. Lu, J. Y. Cui, X. Shi, R. Fathi, J. H. Chen*, Z. Yang*
Two types of isoquinoline scaffolds were successfully constructed in a combinatorial format via the Ugi four-component reaction and the Pd-catalyzed intramolecular Heck reaction, starting from readily available starting materials.
4.Synthesis of a Novel C2-Symmetric Thiourea and Its Application in the Pd-Catalyzed Cross-Coupling Reactions with Arenediazonium Salts under Aerobic Conditions
M. J. Dai, B. Liang, C. H. Wang, J. H. Chen*, Z. Yang*
A novel thiourea-based C2-symmetric ligand was synthesized, and its application in the palladium-catalyzed Heck and Suzuki coupling reactions of arenediazonium salts was evaluated. The reactions, which were performed at room temperature, without added base, and under aerobic conditions, produced product in 4 h with good yield. The corresponding arenediazonium salts were easily generated in one step from anilines.

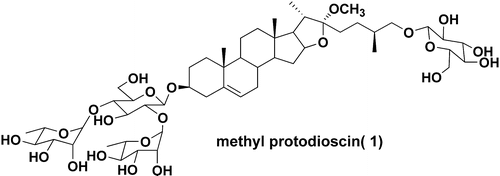
3.Total Synthesis of Methyl Protodioscin: A Potent Agent with Anti-tumor Activity
M. S. Cheng, Q. L. Wang, Q. Tian, H. Y. Song, Y. X. Liu, Q. Li, X. Xu, H. D. Miao, X. S. Yao*, Z. Yang*
Methyl protodioscin (1), otherwise known as 3-O-[α-l-rhamnopyranosyl-(1→2)-{α-l-rhamnopyranosyl-(1→4)}-β-d-glucopyranosyl]-26-O-[β-d-glucopyranosyl]-22-methoxy-25(R)-furost-5-ene-3β,26-diol, has been synthesized for the first time from diosgenin through nine steps in an overall yield of 7.8%.
2.Development of Thiourea-Based Ligands for the Palladium-Catalyzed Bis(methoxycarbonylation) of Terminal Olefins
M. J. Dai, C. H. Wang, J. Xiang, B. Liang, T. P. Luo, J. H. Chen*, Z. Yang*
Eur. J. Org. Chem. 2003, 4346.
Thiourea-based ligands were evaluated for the palladium-catalyzed bis(methoxycarbonylation) of terminal olefins. The usefulness of these ligands for this reaction is demonstrated by their stability to oxidizing agents, and their superiority in preventing palladium precipitation and double-bond isomerization. (© Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2003)