News&Event
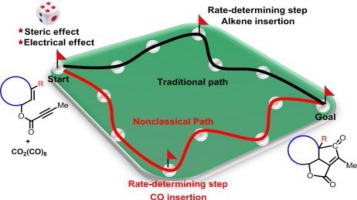
132.Theoretical prediction on the reactivity of the Co-mediated intramolecular Pauson-Khand reaction for constructing bicyclo-skeletons in natural products
LeiZhu, ZheyuanWang, SongLiu, TaoZhang, ZhenYang, RuopengBai, YuLan
Chin. Chem. Lett. 2019, 30 (4), 889-894
The Co2(CO)8-mediated intramolecular Pauson-Khand reaction is an efficient approach for constructing polycyclic skeletons. Recently, some of us reported a series of this type reactions involving sterically-hindered enynes for synthesizing natural products with reasonable reaction rates and yields. However, the reason for the high reactivity of the reaction remains unclear. We employed density functional theory calculations to clarify the mechanism and reactivity for this reaction. In contrast with chain olefin reactants, CO insertion is considered to be the rate-determining step for the overall Pauson-Khand reaction of cyclooctene derivatives. The reduced activation free energy for the alkene insertion step is attributed to: i) the electron-withdrawing group in close proximity to the CC triple bond enhancing the reactivity of the alkyne moiety; ii) lower steric hindrance during alkene insertion when using the cyclooctene derivative. The effect of the substituent on the Co2(CO)8-mediated intramolecular Pauson-Khand reaction was then investigated. Internal alkenes exhibit lower reactivity than terminal alkenes because of the steric hindrance introduced by the substituted group. The cis internal alkene exhibits higher reactivity than the trans internal alkene. An ester group in close proximity to the CC triple bond significantly enhances the reactivity.
131.Stereoselective Pd-Catalyzed Decarboxylative Allylation: Assembly of Highly Functionalized Allylic Amines Bearing a Quaternary Center
Linlin Shi, Yingdong He, Yuanyuan Chang, Nan Zheng, Zhen Yang*, and Jianxian Gong*
Org. Lett. 2019, 21(9), 3077-3080
Here, we report a practical and reliable methodology to direct construction of tri- and tetrasubstituted olefins bearing an allylic amine, with the concomitant construction of the sterically congested quaternary stereocenter through stereoselective palladium-catalyzed cascade decarboxylation of vinyloxazolidinones.
130.The Journey of Schinortriterpenoid Total Syntheses
Zhen Yang*
Acc. Chem. Res., 2019, 52 (2), 480–491
Plants in the Schisandraceae family are important components of the traditional Chinese herbal medicines and are often used to treat various illnesses. Therefore, these Schisandraceae plants are valuable sources for the discovery of new chemical entities for novel therapeutic development. Considerable progress has been made in the identification of bioactive and structurally novel triterpenoids from the Schisandraceae family in the past two decades. In particular, Sun and co-workers have successfully isolated over 100 nortriterpenoids from the Schisandraceae family. Some of these nortriterpenoids have strong inhibitory activities toward hepatitis, tumors, and HIV-1. However, the natural scarcity of these nortriterpenoids in the Schisandraceae plants has hampered their isolation and further biomedical development, and their biosynthesis has not been fully elucidated. It is therefore important and urgent to develop efficient and streamlined total syntheses of these medicinally important nortriterpenoids. Such syntheses will provide sufficient materials for detailed biological studies as well as new synthetic analogues and probe molecules to improve their biological functions and elucidate their mode of actions. However, because of their structural novelty and complexity, the total syntheses of these nortriterpenoid natural products present a significant challenge for synthetic chemists, despite the progress made in organic synthesis, particularly total synthesis, in the 20th century and since the beginning of the 21st century. New synthetic methodologies and strategies therefore need to be invented and developed to facilitate the total syntheses of these nortriterpenoid natural products. With this in mind, our group has spent the last 15 years, ever since the isolation of micrandilactone A (1) by Sun and co-workers in 2003 (Sun et al. Org. Lett. 2003, 5, 1023−1026), working on synthetic studies with a view to developing methods and strategies for the total syntheses of schinortriterpenoids. Enabling methods such as a thiourea/Pd-catalyzed alkocycarbonylative annulation and a thiourea/Co-catalyzed Pauson–Khand reaction have been developed under these circumstances to form the key ring systems and stereocenters of these complex target molecules. These methodological advances have led us to the first total syntheses of schindilactone A (2), lancifodilactone G acetate (6a), 19-dehydroxyarisandilactone A (9), and propindilactone G (10) with diverse structural features via a branching-oriented strategy. The chemistry developed during our total synthesis campaign has not only helped us to deal with various challenges encountered in the syntheses of the four target molecules, but has also opened up new avenues for synthesizing other naturally occurring schinortriterpenoids and their derivatives, which will likely result in molecules with improved biological functions and tool compounds to enable elucidation of their mechanism of actions or potential cellular targets. This Account highlights the chemistry evolution of our schinortriterpenoid syntheses.
129.Formal Total Synthesis of Hybocarpone Enabled by Visible-Light-Promoted Benzannulation
Wei Chen, Renyu Guo, Zhen Yang*, and Jianxian Gong*
J. Org. Chem., 2018, 83 (24), pp 15524–15532
The formal total synthesis of hybocarpone was achieved in eight steps from commercially available 1,2,4-trimethoxybenzene. Key transformations include a visible-light-promoted benzannulation to construct the key α-naphthol intermediate and a modified CAN-mediated dimerization/hydration cascade sequence to generate the vicinal all-carbon quaternary centers in a stereocontrolled manner. The total synthesis of boryquinone was also achieved in seven steps.
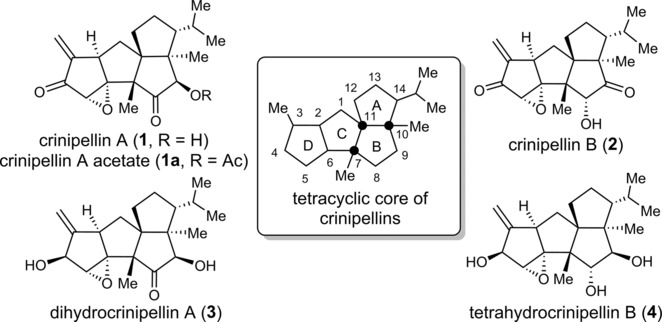
128.Total Syntheses of Crinipellins Enabled by Cobalt‐Mediated and Palladium‐Catalyzed Intramolecular Pauson–Khand Reactions
Zhihui Huang, Dr. Jun Huang, Yongzheng Qu, Weibin Zhang, Prof. Dr. Jianxian Gong*, Prof. Dr. Zhen Yang*
Angew. Chem. Int. Ed. 2018, 57(28), 8744 –8748
• Highlighted in Synfacts, 2018, 14, 788.
Efficient total syntheses of the naturally occurring, potent antibiotic compounds (−)‐crinipellin A and (−)‐crinipellin B are described. The key advanced intermediate, a fully functionalized tetraquinane core, was constructed by a novel thiourea/palladium‐catalyzed Pauson–Khand reaction. This intermediate can serve as a common intermediate for the collective total synthesis of other members of the crinipellin family.