News&Event
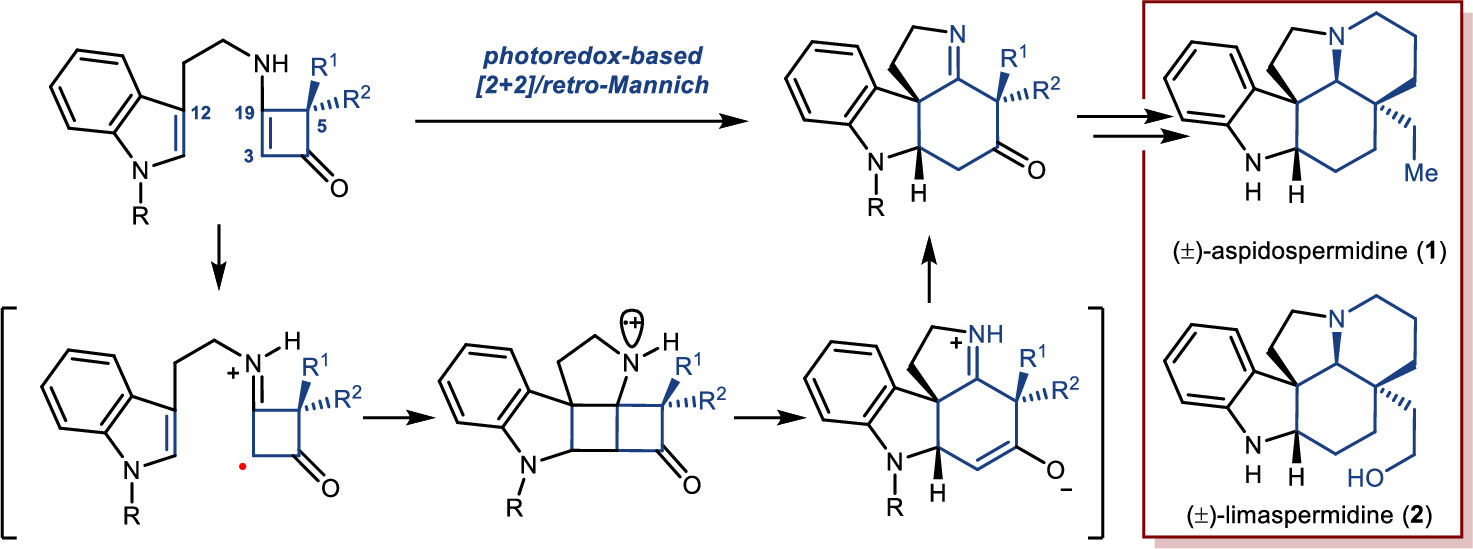
172.Development of a Strategy for the Total Synthesis of Aspidosperma Alkaloids via the Cyclobutenone-Based PET-Initiated Cationic Radical-Driven [2+2]/Retro-Mannich Reaction
Jianyu Long, Rudong Liu, Xinpeng Mu, Zhilin Song, Zhongchao Zhang,* and Zhen Yang*
Org. Lett. 2024, 26(15), 2960–2964
A novel strategy for the synthesis of Aspidosperma alkaloids has been achieved via a photoredox-initiated [2+2]/retro-Mannich reaction of tryptamine-substituted enaminones as a key step. The developed chemistry has been applied to the construction of the core tetracycle of Aspidosperma alkaloids (±)-aspidospermidine and (±)-limaspermidine.
171.Total Synthesis of Penicibilaenes Enabled by a Tandem Double Conia-ene Type Reaction
Zheyuan Wang, Zhilin Song, Jun Huang,* and Zhen Yang*
J. Am. Chem. Soc. 2024, 146(7), 4363–4368
The total syntheses of penicibilaenes A and B are described. The key step is the tBuOK/DMSO-mediated tandem 5-exo–dig Conia-ene type reaction and 6-exo–dig Conia-ene type reaction to install the tricyclic [6.3.1.01,5] dodecane core of penicibilaenes from dibutynyl cyclohexanone in a single step, together with a sequence of copper-mediated conjugate addition and Crabtree’s hydrogenation to forge the stereogenic centers at C5 and C2, respectively.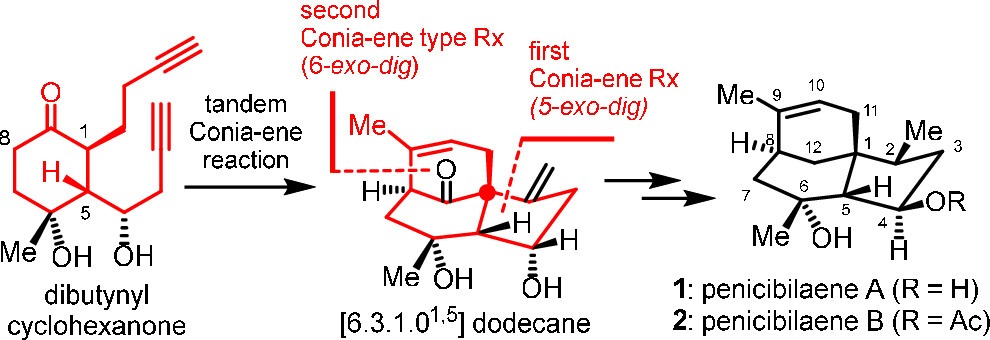
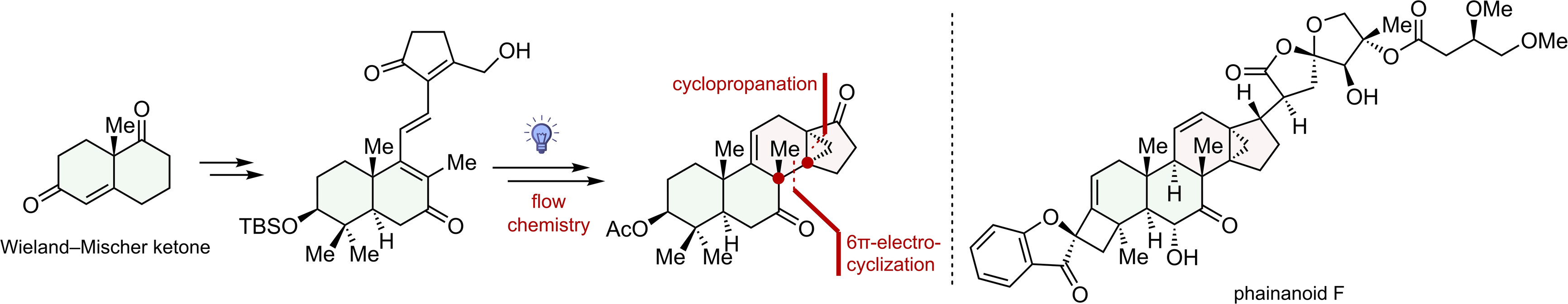
170.Synthesis towards Phainanoid F: Photo-induced 6π- Electrocyclization for Constructing Contiguous All-Carbon Quaternary Centers
Hao-Yuan Liu, Zhen-Yu Zhang, Yi-Ke Zhou, Jia-Hua Chen, Zhen Yang,* and Yuan-He Li*
Chem Asian J.2023,18, e20230062
In this paper, we report an efficient strategy for synthesizing the DEFGH rings of phainanoid F. The key to the construction of the 13,30-cyclodammarane skeleton of the molecule was a photo-induced 6π-electrocyclization and a homoallylic elimination. Notably, this is a rare example of using electrocyclization reaction to simultaneously construct two vicinal quaternary carbons in total synthesis. The strategy outlined here forms the basis of our total synthesis of Phainanoid F, and it could also serve as a generally applicable approach for synthesizing other natural products containing similar 13,30-cyclodammarane skeletons.
169.Regioselective Hydroxylation of Flavonoids by Transition-Metal-Catalyzed C−H Bond Oxidation
Shu-Min Lu, Chao Chen, Chang Liu, Rudong Liu, Jia-Hua Chen,* Zhongchao Zhang,* and Zhen Yang*
Org. Lett. 2023, 25(13), 2264–2269
Regioselective synthesis of 5,6,7-trihydroxyl and 5,7,8-trihydroxyl flavones has been achieved via a transition-metal-catalyzed C–H oxidation as the key step using naturally enriched 5,7-dihydroxyl flavone. The developed chemistry was applied to the synthesis of the naturally occurring and biologically active flavonoids wogonin (2), oroxylin A (3), and their glycosylated derivatives (4 and 5) as potential carnitine palmitoyltransferase 1 activators.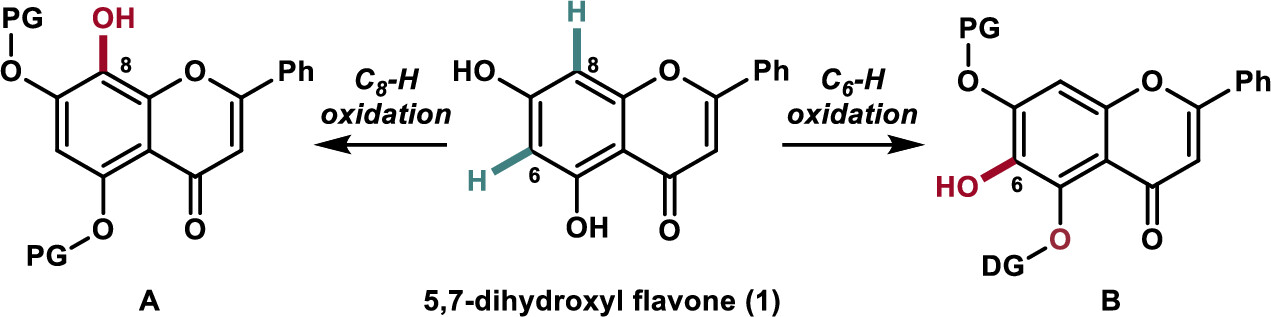
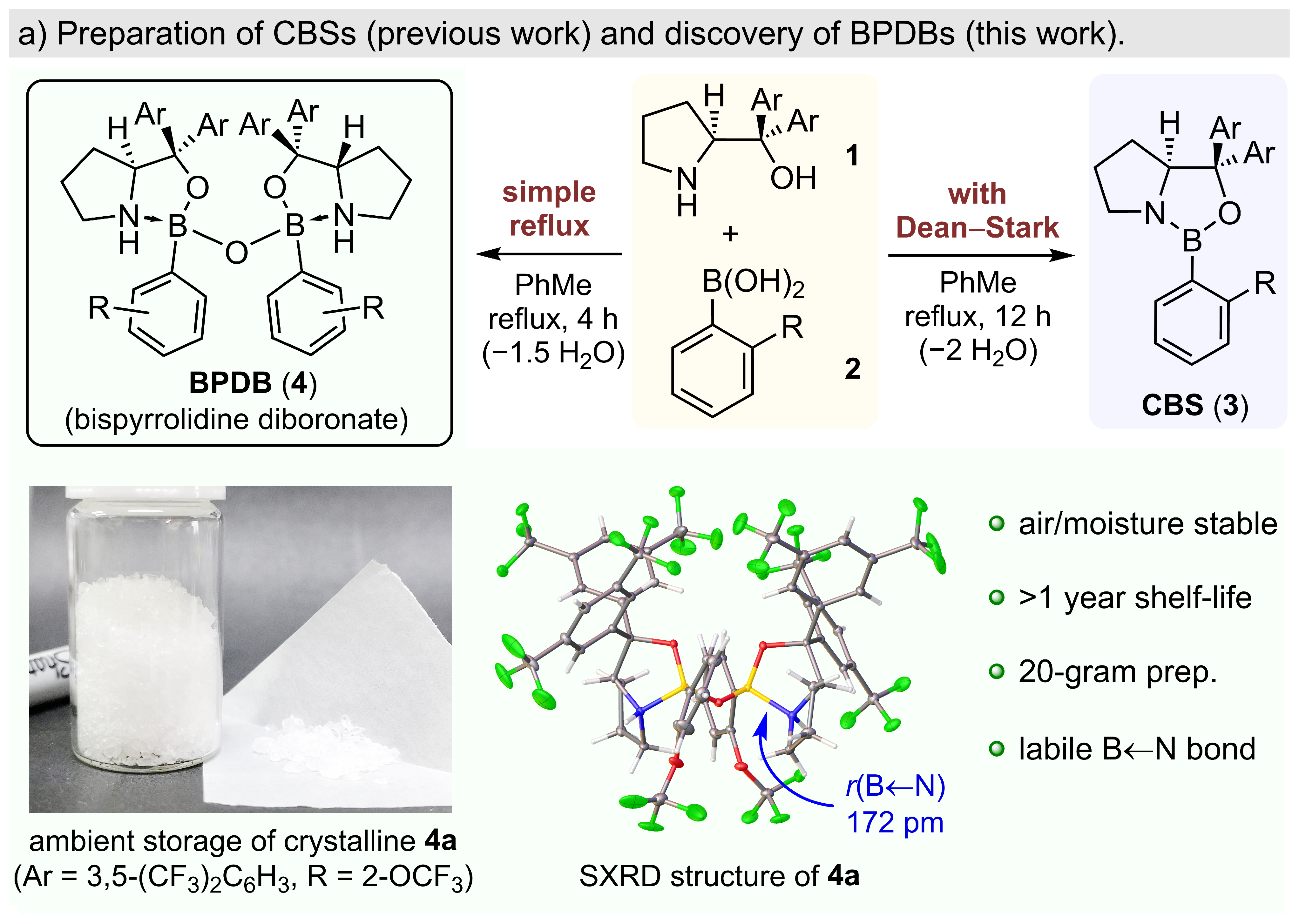
168.Highly Stereoselective Diels-Alder Reactions Catalyzed by Diboronate Complexes
Yuan-He Li, Su-Lei Zhang, Yong Lu, Bo Xiao, Tian-Yu Sun, Qian-Qian Xu, Jia-Hua Chen,* Zhen Yang*
Angew. Chem. Int. Ed. 2023, 62(33)
A highly enantioselective catalytic system for exo-Diels–Alder reactions was developed based on the newly discovered bispyrrolidine diboronates (BPDBs). When activated various Lewis or Brønsted acids, BPDBs could catalyze highly stereoselective asymmetric exo-Diels–Alder reactions of monocarbonyl-based dienophiles. When 1,2-dicarbonyl-based dienophiles are used, the catalyst can sterically distinguish between the two binding sites, which leads to highly regioselective asymmetric Diels–Alder reactions. BPDBs can be prepared as crystalline solids on a large scale and are stable under ambient conditions. Single-crystal X-ray analysis of the structure for acid-activated BPDB indicated that activation involves breakage of a labile N→B bond.