News&Event
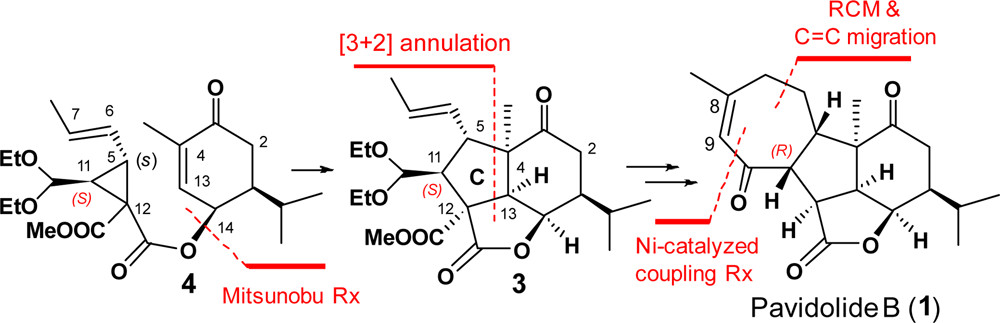
121. Enantioselective Total Synthesis of (−)-Pavidolide B
Peng-Peng Zhang, Zhi-Ming Yan, Yuan-He Li, Jian-Xian Gong*, and Zhen Yang*
J. Am. Chem. Soc., 2017, 139 , 13989–13992
• Second Most Read Articles in JACS 2017/10.
• Highlighted in Synfacts, 2017, 13, 1233.
• Highlighted in Org. Chem. Highlights 2017, April 2.
• Highlighted in ChemistryWorld, 2017, Nov, 1.
• Highlighted in Chin. J. Org. Chem. 2018, 38, 282.
The enantioselective synthesis of (−)-pavidolide B (1) was achieved in a linear sequence of 10 steps. The key steps are (a) an enantioselective organocatalytic cyclopropanation; (b) a radical-based cascade annulation for the regio- and diastereo-selective synthesis of the highly functionalized lactone 3 bearing the characteristic tricyclic core and seven contiguous stereocenters; (c) a sequential ring-closing metathesis reaction and a RhCl3-catalyzed double bond isomerization to form the seven-membered D ring of (−)-pavidolide B.

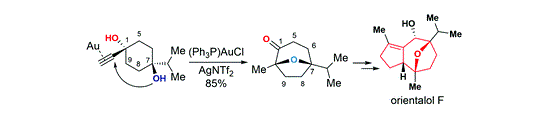
120.Total synthesis of orientalol F via gold-catalyzed cycloisomerization of alkynediol
Org. Chem. Front., 2017, 4, 2296-2300
The total synthesis of orientalol F has been achieved starting from 1,4-dioxaspirodecan-8-one 11 in 13 steps. The key steps in this synthesis feature: (1) gold-catalyzed tandem cycloisomerization of alkynediol 10 for the formation of its seven-membered oxa-bridged bicyclic skeleton 9 of orientalol F, (2) visible-light-promoted organocatalytic aerobic oxidation of silyl enol ether 16 to enone 17, (3) Barbier-type butenylation for the diastereoselective synthesis of allylic alcohol 18 from enone 17, and (4) substrate-controlled Pd-catalyzed hydrogenation of 20 for the stereoselective installation of the C1 stereogenic center of 8.
119.Diastereoselective Synthesis of Diquinanes and Triquinanes Bearing Vicinal Quaternary Carbon Stereocenters from Acyclic Allene-based Precursors via a Cascade Reaction
Shuang Li, Pengpeng Zhang, Yuanhe Li, Shumin Lu, Jianxian Gong*, and Zhen Yang*
A cascade benzenethiol-mediated intramolecular [3 + 2] cycloaddition reaction between an allene and an α,β-unsaturated aldehyde or ester is developed for the diastereoselective synthesis of [3.3.0] bicyclic system bearing two quaternary atoms at their bridgehead positions. Notably, these structurally complex systems can be found in a wide range of natural products.

118.Asymmetric total synthesis of (−)-perforanoid A
Chao, Lv ; Qian, Tu ; Jianxian, Gong*; Xiaojiang, Hao*; Zhen, Yang*
Asymmetric total synthesis of (−)-perforanoid A, a novel limonoid isolated from the leaves ofHarrisonia perforata, has been achieved. The key features of our total synthesis include the Rh-catalyzed intramolecular Pauson–Khand reaction of an allene and an alkene, the Pd-catalyzed lactonization of an allylic alcohol with a vinyl ether, and the enantioselective alkenylation of 3-furaldehyde.
117.A rhodium-catalyzed tandem reaction of N-sulfonyl triazoles with indoles: access to indole-substituted indanones
An efficient strategy for the synthesis of structurally diverse indole-substituted indanones via a rhodium(II)-catalyzed tandem reaction of N-sulfonyltriazoles with indoles was developed. The reaction involves rhodium(II)-catalyzed denitrogenation of the N-sulfonyltriazoles to form an oxonium ylide, followed by nucleophilic addition of the indoles and subsequent skeletal rearrangement. This strategy provides straightforward access to indanone frameworks bearing quaternary carbon centers.

