News&Event
23.Efficient Synthesis of Maleimides and Carbazoles via Zn(OTf)2-Catalyzed Tandem Annulations of Isonitriles and Allenic Esters
Y. Z. Li, H. X. Zou, J. X. Gong, J. Xiang, T. P. Luo, J. M. Quan, G. X. Wang*, and Z. Yang*
Lewis acid Zn(OTf)2-catalyzed tandem annulations of isonitriles and allenic esters which lead to efficient and flexible syntheses of a range of biologically significant maleimides and carbazoles and related compounds are reported. A mechanistic rationale is proposed to account for the observed reactivity.

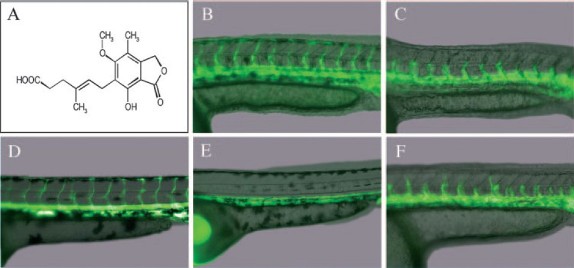
22.Mycophenolic Acid is a Potent Inhibitor of Angiogenesis
X. Wu, H. Zhong, J. Song, R. Damoiseaux, Z. Yang*, S. Lin*
Arterioscler Thromb Vasc Biol. 2006, 26, 2414.
Angiogenesis is necessary for the vascularization of a tumor, providing essential nourishment for tumor growth, and the progression and metastasis of cancer cells.
21.Synthetic Study of 1,3-Butadiene-Based IMDA Approach to Construct a [5-7-6] Tricyclic Core and Its Application to the Total Synthesis of C8-epi-Guanacastepene O
C. C. Li, C. H. Wang, B. Liang, X. H. Zhang, L. J. Deng, S. Liang, J. H. Chen*, Y. D. Wu*, Z. Yang*
J. Org. Chem., 2006, 71, 6892.
An efficient intramolecular Diels−Alder (IMDA) strategy for the construction of the [5−7−6] tricyclic core (18) of guanacastepenes has been developed from cis– and trans-1,3-butadiene-tethered 4-oxopent-2-ynoic acid ethyl esters 10 and 11. This method facilitates the synthesis of C8-epi-guanacastepene O (36) in a very efficient manner.

20.Synthesis of Functionalized Quinolines via Ugi and Pd-Catalyzed Intramolecular Arylation Reactions
Z. B. Ma, Z. Xiang, T. P. Luo, K. Lu, Z. Xu, J. H. Chen*, Z. Yang*
Two types of quinoline scaffolds were constructed in a combinatorial format via the Ugi four-component reaction (U-4CR) and Pd-catalyzed intramolecular arylation reaction. The scope of this two-step synthetic sequence was examined from commercially available and synthetically accessible starting materials.

19.Development of a Concise and Diversity-Oriented Approach for the Synthesis of Plecomacrolides via the Diene-Ene RCM
K. Lu, M. W. Huang, Z. Xiang, Y. Liu, J. H. Chen*, Z. Yang*
A concise synthesis of the core structures of plecomacrolide with ring sizes varying from 16 to 19 atoms was achieved for the first time by the diene−ene ring-closing olefin metathesis reaction. This approach should allow access to the structurally diverse analogues of plecomacrolide.

