News&Event
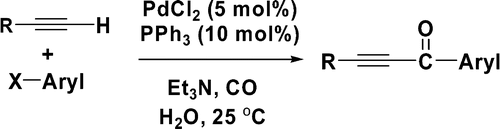
13.Pd-Catalyzed Copper-Free Carbonylative Sonogashira Reaction of Aryl Iodides with Alkynes for the Synthesis of Alkynyl Ketones and Flavones by Using Water as a Solvent
B. Liang, M. W. Huang, Z. J. You, Z. C. Xiong, K. Lu, R. Fathi, J. H. Chen*, Z. Yang*
The Pd-catalyzed copper-free carbonylative Sonogashira coupling reaction to synthesize alkynyl ketones from terminal alkynes and aryl iodides was achieved by using water as a solvent. The reaction was carried out at room temperature under balloon pressure of CO with Et3N as a base. The developed method was successfully applied to the synthesis of flavones.
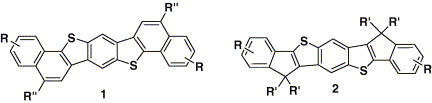
12.Linear C2-Symmetric Polycyclic Benzodithiophene: Efficient, Highly Diversified Approaches and the Optical Properties
C. H. Wang, R. Hu, S. Liang, J. H. Chen. Z. Yang*, J. Pei*
Tetrahedron Lett., 2005, 46, 8153.
Two facile approaches to two new series of the seven-rings fused benzodithiophene-based polycyclic aromatics are developed in good yields.
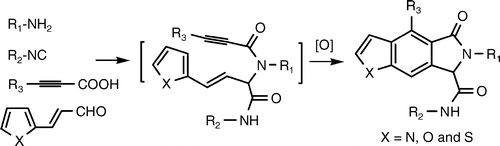
11.A Concise and Diversity-Oriented Strategy for the Synhteis of Benzofurans and Indoles via Ugi and Dields-Alder Reaction
K. Lu, T. P. Luo, Z. Xiang, Z. J. You, R. Fathi, J. H. Chen*, Z. Yang*
A one-pot synthesis of diverse benzofurans and indoles from readily available starting materials was achieved via the sequential Ugi four-component reaction, intramolecular Diels−Alder reaction, and oxidative aromatization.
10.Novel PdII-Mediated Cascade Carboxylative Annulation to Construct Benzo[b]furan-3-carboxylic Acids
Y. Liao*, J. Smith, R. Fathi*, Z. Yang*
Benzo[b]furan-3-carboxylic acid (2) was generated from 1 by forming three new bonds in one step via a PdII-mediated cascade carboxylative annulation. The proposed mechanism was supported by the observation of an unusual acetylation of 1 as a side reaction together with an 18O-labeling study.
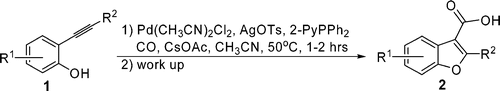
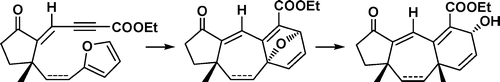
9.Exploring an Expedient IMDA Reaction Approach to Construct the Guanacastepene Core
C. C. Li. S. Liang, X. H. Zhang, Z. X. Xie, J. H. Chen*, Y. D.Wu*, Z. Yang*
Construction of the [5-7-6] tricyclic core of guanacastepenes was attempted by using the intramolecular Diels−Alder (IMDA) reaction and Me3Al-mediated ring opening of the oxabridge as key synthetic steps. The illustrated chemistry demonstrated a synthetic feasibility to build up the framework of guanacastepenes by the IMDA reaction.