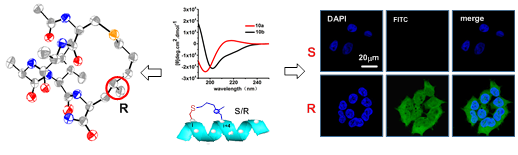
The addition of a precisely positioned chiral center in the tether of a constrained peptide is reported, yielding two
separable peptide diastereomers with significantly different helicity, as supported by circular dichroism (CD) and NMR spectroscopy. Single crystal X-ray diffraction analysis suggests that the absolute configuration of the in-tether chiral center in helical form is R, which is in agreement with theoretical simulations. The relationship between the secondary structure of the short peptides and their biochemical/biophysical properties remains elusive, largely because of the lack of proper controls. The present strategy provides the only method for investigating the influence of solely conformational differences upon the biochemical/biophysical properties of peptides. The significant differences in permeability and target binding affinity between the peptide diastereomers demonstrate the
importance of helical conformation.
This article was published in the “Angewandte Chemie” (Kuan Hu, Hao Geng, Qingzhou Zhang, Qisong Liu, Mingsheng Xie, Chengjie Sun, Wenjun Li, Huacan Lin, Fan Jiang, Tao Wang,* Yun-Dong Wu,* and Zigang Li* An In-tether Chiral Center Modulates the Helicity, Cell Permeability,and Target Binding Affinity of a Peptide. Angew. Chem., Int. Ed.2016,DOI: 10.1002/anie.201602806R1.)
Linking:http://onlinelibrary.wiley.com/doi/10.1002/anie.201602806/full
http://www.x-mol.com/news/3194
http://mp.weixin.qq.com/s?__biz=MzAwOTExNzg4Nw==&mid=2657563176&idx=4&sn=9d936b985b06b7ee7f9702c0e3623315#rd