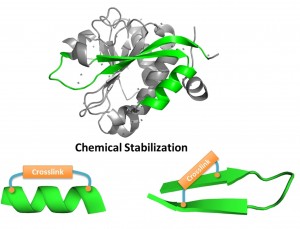
Recently, we developed methods to stabilize peptides into various secondary structures, including a-helix, type III turn and b-hairpin via proper thioether based macrocyclization. These conformationally constrained peptidomimetics confer enhanced biophysical properties and provide a valuable avenue towards clinically-relevant therapeutic molecules. In this personal account, thioether-derived macrocyclization methods developed by our group for stabilization of a-helix, type-III b turn and b-hairpin conformations are discussed.
Link:http://onlinelibrary.wiley.com/doi/10.1002/tcr.201600137/full