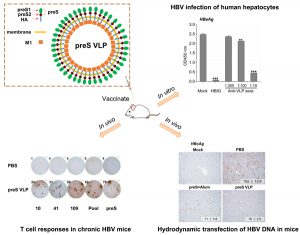
The preS antigen of hepatitis B virus (HBV) corresponds to the N-terminal polypeptide in the large (L) antigen in addition to the small (S) antigen. The virus-like particle (VLP) of the S antigen is widely used as a vaccine to protect the population from HBV infection. The presence of the S antigen and its antibodies in patient blood has been used as markers to monitor hepatitis B. However, there is very limited knowledge about the preS antigen. We generated a preS VLP that is formed by a chimeric protein between preS and hemagglutinin (HA), and the matrix protein M1 of influenza virus. The HBV preS antigen is displayed on the surface of preS VLP. Asn112 and Ser98 of preS in VLP were found to be glycosylated and O-glycosylation of Ser98 has not been reported previously. The preS VLP shows a significantly higher immunogenicity than recombinant preS, eliciting robust anti-preS neutralizing antibodies. In addition, preS VLP is also capable of stimulating preS-specific CD8+ and CD4+ T cell responses in Balb/c mice and HBV transgenic mice. Furthermore, preS VLP immunization provided protection against hydrodynamic transfection of HBV DNA in mice. The data clearly suggest that this novel preS VLP could elicit robust immune responses to the HBV antigen, and can be potentially developed into prophylactic and therapeutic vaccines.
Link: https://www.sciencedirect.com/science/article/pii/S0166354217305351