News&Event
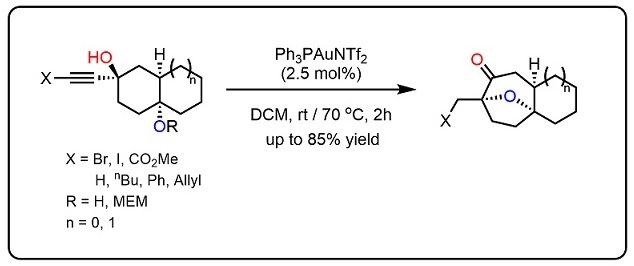
99.Towards a general diastereoselective route to oxabicyclo[3.2.1]octanes via a gold-catalysed cascade reaction
Junkai Fu, Yueqing Gu, Hao Yuan, Tuoping Luo, Song Liu, Yu Lan*, Jianxian Gong*, Zhen Yang*
Nat. Commun., 2015, 6:8617. DOI: 10.1038/ncomms9617 (2015)
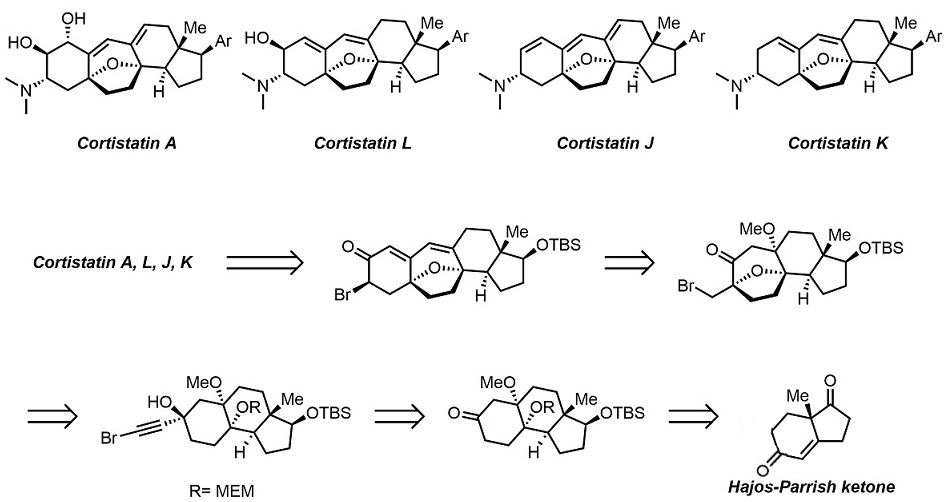
The development of an efficient diastereoselective synthesis of the oxabicyclo[3.2.1]octane ring system bearing two oxygenated quaternary chiral centres represents a significant challenge. This motif can be found in a wide range of natural products with significant biological activities. Here we report the synthesis of such kind of scaffold using a cyclohexane-trans-1,4-diol with an alkyne side chain in the presence of Au(I) catalyst. This is a domino process in which two C–H, two C–O and one C–C bond is assembled through a sequence of cyclization/semi-pinacol rearrangements. This strategy has been successfully applied to the asymmetric formal total synthesis of (+)-cortistatins.
The new review article on NPR was published
Our new reivew article regarding Direct construction of vicinal all-carbon quaternary stereocenters in natural product synthesis was published as Cover Paper on Nat. Prod. Rep., 2015,32, 1584
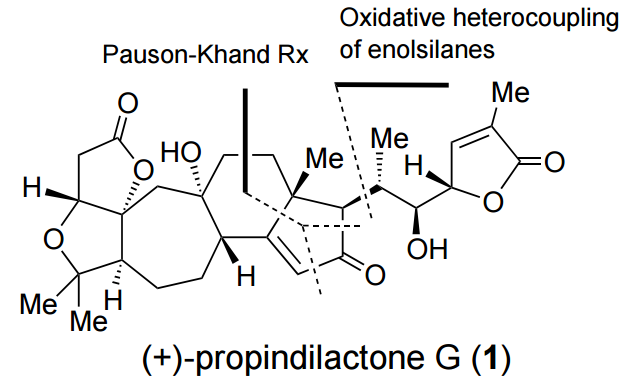
The total synthesis of propindilactone G was highlighted by Synfacts!
The total synthesis of propindilactone G was highlighted in Synfacts, 2015, 11, 1022.
The seven-membered carbocycle F was accessed via a silver-mediated cationic ring expansion. Further salient features of this synthesis include a Pauson–Khand cyclization and an oxidative heterocoupling of silyl enol ethers J and K.
98.Direct construction of vicinal all-carbon quaternary stereocenters in natural product synthesis
Rong Long, Jun Huang, Jianxian Gong* and Zhen Yang*
Nat. Prod. Rep., 2015,32, 1584
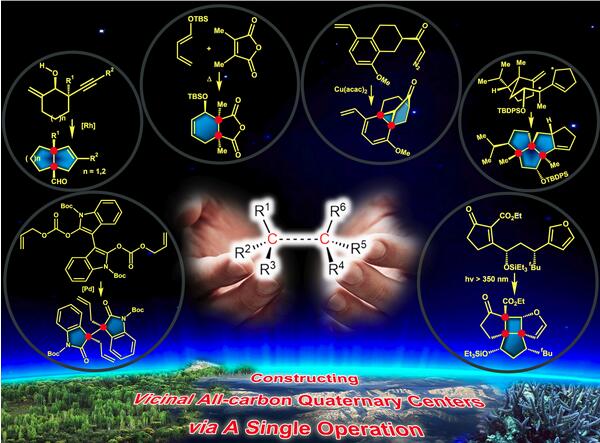
Molecules containing vicinal all-carbon quaternary stereocenters are found in many secondary metabolites, and they exhibit a variety of biological and pharmacological activities. However, the construction of such a structural motif remains a significant challenge in natural product synthesis. Only in recent years have considerable efforts been made to construct vicinal quaternary stereocenters in a single-step operation. In this review, we focus on the different types of methods that have been successfully used in the total synthesis of natural products. Based on the classified reactions for the simultaneous generation of vicinal all-carbon quaternary stereocenters, the total syntheses of the natural products are discussed, placing emphasis on the diastereoselective preparation of vicinal quaternary carbon centers and the subsequent total syntheses.
97.Gold-Catalyzed Intramolecular Tandem Cyclization of Indole-Ynamides: Diastereoselective Synthesis of Spirocyclic Pyrrolidinoindolines
Nan Zheng, Yuan-Yuan Chang, Li-Jie Zhang, Jian-Xian Gong,* and Zhen Yang*
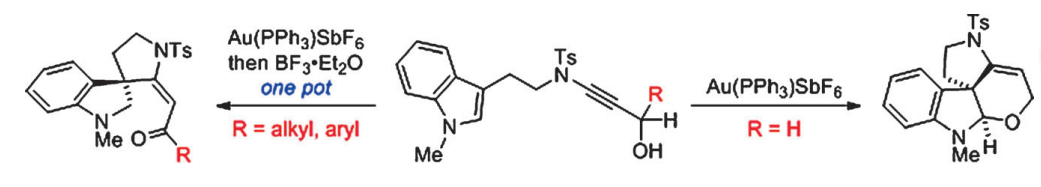
A gold-catalyzed intramolecular tandem cyclization of indole-ynamide affords tetracyclic spirocyclic pyrrolidinoindoline bearing an all-carbon quaternary stereocentre in a single step; however, when the reaction was carried out in the presence of BF3⋅Et2O, the corresponding tricyclic spirocyclic pyrrolidinoindoline-based enones are produced through a key 1,5-hydride shift. The developed chemistry provides a diastereoselective and straightforward entry to structurally diverse polycylic pyrrolidinoindolines from indole-ynamides in one-pot reactions under mild conditions.