News&Event
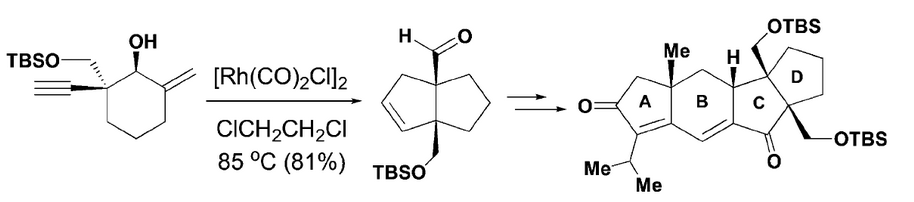
94.Diastereoselective Synthesis of Cyclopentanoids: Applications to the Construction of the ABCD Tetracyclic Core of Retigeranic Acid A [Inside Back Cover]
Junlin Zhang, Xiao Wang, Shuang Li, Dian Li, Song Liu, Prof. Dr. Yu Lan*, Dr. Jianxian Gong*, Prof. Dr. Zhen Yang*
A concise and efficient approach for the construction of the tetracyclic carbon skeleton of retigeranic acid A is described. The key transformations include a novel Rh-catalyzed [3+2] cycloaddition of enyol to afford cyclopentanoid E, bearing two contiguous quaternary stereocenters at the bridgehead positions, and an intramolecular Pauson–Khand reaction to construct the advanced tetracyclic core structure of retigeranic acid A.
Long R., Huang J., Fu J.-K., Zhang J.-L. and Zhang W.-B. have successfully defended their dissertations
Long Rong, Huang Jun, Fu Jun-kai, Zhang Jun-lin and Zhang Wei-bin have successfully defended their dissertations and shall be conferred the doctor’s degree. Congratulations!
Fu Jun-kai is also been titled as “Outstanding Graduates of Peking University” due to his excellent academic performance. Congratulations!
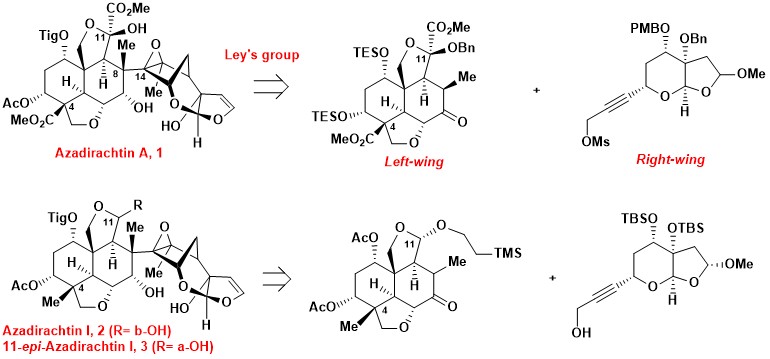
Synthetic progress toward 11-epi-azadirachtin I
Azadirachtin A is a highly oxygenated tetranortriterpenoid isolated from the neem tree Azadirachta Indica A. Juss (Meliaceae). Azadirachtin A is a powerful insect repellent with low mammalian toxicity, however, there is limited structure-activity relationship information and it has an elusive mechanism-of-action.2 Various azadirachtin congeners possess remarkable antifeedant activity, such as azadirachtin I acts against Spodoptera litura with similar potency to azadirachtin A. The anti-insect properties of these azadirachtin-type limonoids are less well studied, partially due to limited availability.
Azadirachtin A possesses a number of intriguing structural elements, such as a highly oxygenated scaffold, sixteen contiguous chiral centers including seven quaternary ones, and a crowded C8–C14 bond. These fascinating and highly complex structural features have challenged as well as inspired synthetic chemists for decades, culminating in the “relay total synthesis” of azadirachtin A by Ley et al. One key to Ley’s success is the strategic disconnection of the C8–C14 bond, leading to the left- and right-wing (4–5) fragments.
Synthetic progress toward 11-epi-azadirachtin I was reported as two related paper which were published on Org. Lett.. For more details, please see the paper online: https://web.pkusz.edu.cn/yang/93/ and https://web.pkusz.edu.cn/yang/92/.
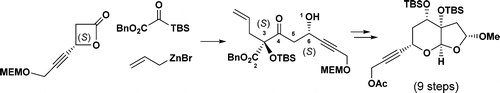
93. Synthetic Progress toward Azadirachtins. 2. Enantio- and Diastereoselective Synthesis of the Right-Wing Fragment of 11-epi-Azadirachtin I
Ceheng Tan, Wei Chen, Xinpeng Mu, Qi Chen, Jianxian Gong *, Tuoping Luo *, and Zhen Yang
A stereoselective three-component coupling reaction of allylzinc bromide, silyl glyoxylate, and a β-lactone has been developed. This has been successfully applied to the enantio- and diastereoselective synthesis of the fully functionalized furopyran moiety of azadirachtins.
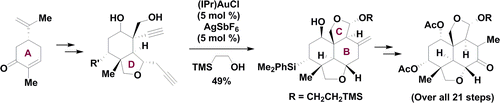
92. Synthetic Progress toward Azadirachtins. 1. Enantio- and Diastereoselective Synthesis of the Left-Wing Fragment of 11-epi-Azadirachtin I
Hang Shi, Ceheng Tan, Weibin Zhang, Zichun Zhang, Rong Long, Tuoping Luo *, and Zhen Yang *
A highly enantio- and diastereoselective synthesis of the left-wing fragment of 11-epi-azadirachtin I characterized with the pairwise use of palladium- and gold-catalyzed cascade reactions is presented. By enlisting a sequence of stereocontrolled transformations, our 21-step route established the stereocenters of the left-wing fragment from one chiral starting material, (−)-carvone, which would significantly facilitate the synthetic studies of the azadirachtin-type limonoids.