News&Event
157.Stereoselective Synthesis of (±)-Cephanolide B
Anding Li, Ziru He, Bingyan Liu, Zhen Yang*, and Zichun Zhang*
Org. Lett. 2021, 23,(23), 9237–9240
A concise and stereoselective total synthesis of (±)-cephanolide B was achieved in 15 steps. The key steps in the synthesis were as follows: (i) an intermolecular Diels–Alder reaction followed by lactonization to form the oxabicyclo[2.2.2]octane DE ring; (ii) a tandem reaction, featuring an intramolecular Pauson–Khand reaction, a 6π-electrocyclization, and an oxidative aromatization by O2, to construct the ABC-tricyclic rings (6-5-6); and (iii) a phthaloyl peroxide-mediated arene oxygenation to install the C-13 phenol group.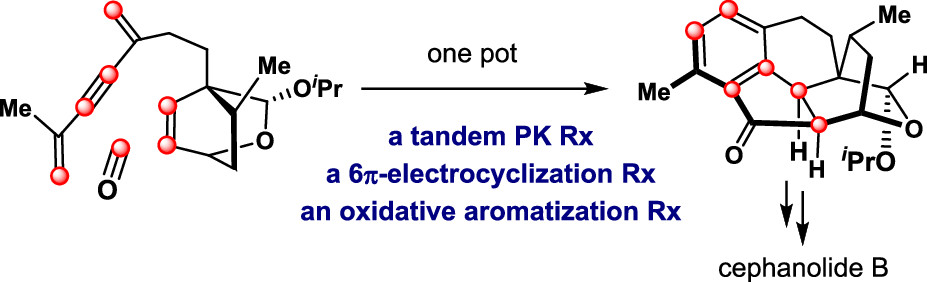
156.Synthetic Strategy for Construction of Highly Congested Tetracyclic Core (6–5–7–4) of Harziane Diterpenoids
Qian Tu, Zheyuan Wang, Zhongchao Zhang, Jun Huang*, and Zhen Yang*
Org. Lett. 2021, 23(11), 4088–4093
The structurally intriguing tetracyclic core of complex harziane diterpenoid was constructed in 14 steps from commercially available 3-ethoxycyclohex-2-en-1-one. The key steps were a Mn/Cu-mediated oxidative 1,3-dicarbonyl radical cascade cyclization reaction, which diastereoselectively formed the core of dimethylbicyclo[3.2.1]octane structure, and a Au-catalyzed diastereoselective formal [2 + 2] cycloaddition for construction of the harziane diterpenoid tetracyclic framework. The developed method paves the way for achieving total synthesis of this type of complex natural product.
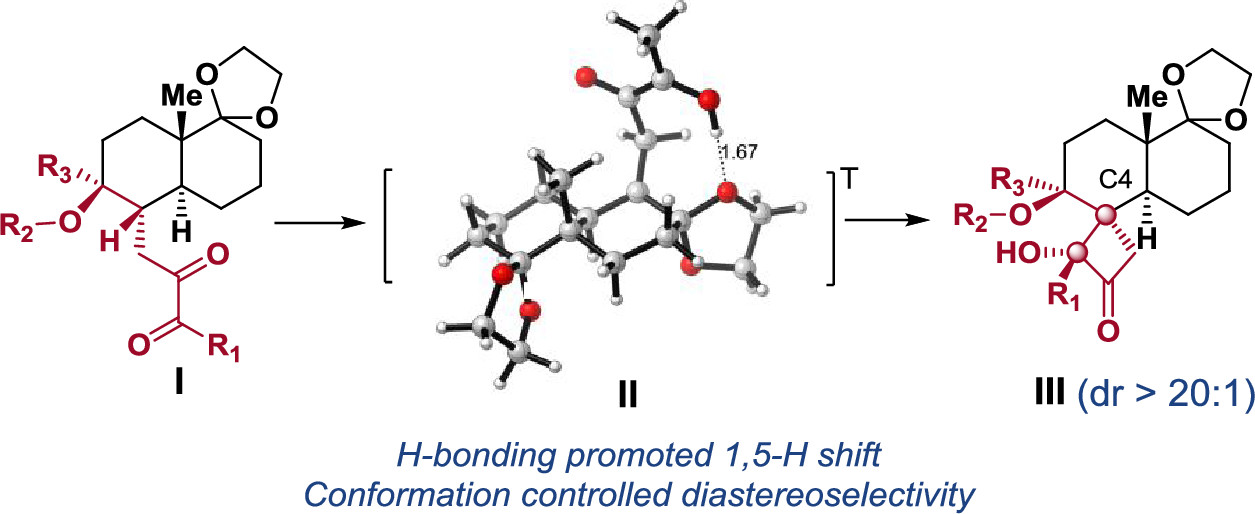
155.Stereoselective Synthesis of trans-Decalin-Based Spirocarbocycles via Photocyclization of 1,2-Diketones
Sijia Chen, Zhongchao Zhang, Chongguo Jiang, Chunbo Zhao, Haojie Luo, Jun Huang*, and Zhen Yang*
ACS Omega 2021, 6(29), 18848–18859
Diastereoselective synthesis of the trans-decalin-based α-hydroxyl butanone spirocarbocycles bearing all-carbon quaternary stereogenic centers has been achieved via Norrish–Yang photocyclization of trans-decalin-substituted-2,3-butanediones using daylight. Density functional theory (DFT) calculations suggest that this diastereoselective reaction is affected by both substrate conformation and intramolecular hydrogen bonds. The developed chemistry could be applied to synthesizing the derivatives of the trans-decalin-based biologically important natural products.
154.Synthesis of Desacyl Furanmonogones A and B
Dian Li, Jinfeng Yang, Bingyan Liu, Jianxian Gong,* and Zhen Yang*
Org. Lett. 2021, 23(12), 4532–4537
A strategy for the stereoselective synthesis of desacyl furanmonogones A and B has been achieved. The key steps in this synthesis are (1) an Fe(ClO4)3-mediated oxidative radical cyclization for construction of a cis-fused [5–6]-bicyclic core with a bridged lactone substitute, (2) a phosphorane-mediated rearrangement to convert the cis-fused [5–6]-bicyclic core to the corresponding trans-fused [5–6]-bicyclic core, and (3) a Au-catalyzed cascade reaction for formation of the 4,5-seco-3(2H)-furanone motif.