Publications
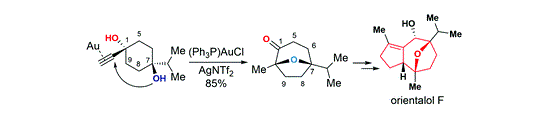
120.Total synthesis of orientalol F via gold-catalyzed cycloisomerization of alkynediol
Org. Chem. Front., 2017, 4, 2296-2300
The total synthesis of orientalol F has been achieved starting from 1,4-dioxaspirodecan-8-one 11 in 13 steps. The key steps in this synthesis feature: (1) gold-catalyzed tandem cycloisomerization of alkynediol 10 for the formation of its seven-membered oxa-bridged bicyclic skeleton 9 of orientalol F, (2) visible-light-promoted organocatalytic aerobic oxidation of silyl enol ether 16 to enone 17, (3) Barbier-type butenylation for the diastereoselective synthesis of allylic alcohol 18 from enone 17, and (4) substrate-controlled Pd-catalyzed hydrogenation of 20 for the stereoselective installation of the C1 stereogenic center of 8.
119.Diastereoselective Synthesis of Diquinanes and Triquinanes Bearing Vicinal Quaternary Carbon Stereocenters from Acyclic Allene-based Precursors via a Cascade Reaction
Shuang Li, Pengpeng Zhang, Yuanhe Li, Shumin Lu, Jianxian Gong*, and Zhen Yang*
A cascade benzenethiol-mediated intramolecular [3 + 2] cycloaddition reaction between an allene and an α,β-unsaturated aldehyde or ester is developed for the diastereoselective synthesis of [3.3.0] bicyclic system bearing two quaternary atoms at their bridgehead positions. Notably, these structurally complex systems can be found in a wide range of natural products.

118.Asymmetric total synthesis of (−)-perforanoid A
Chao, Lv ; Qian, Tu ; Jianxian, Gong*; Xiaojiang, Hao*; Zhen, Yang*
Asymmetric total synthesis of (−)-perforanoid A, a novel limonoid isolated from the leaves ofHarrisonia perforata, has been achieved. The key features of our total synthesis include the Rh-catalyzed intramolecular Pauson–Khand reaction of an allene and an alkene, the Pd-catalyzed lactonization of an allylic alcohol with a vinyl ether, and the enantioselective alkenylation of 3-furaldehyde.
117.A rhodium-catalyzed tandem reaction of N-sulfonyl triazoles with indoles: access to indole-substituted indanones
An efficient strategy for the synthesis of structurally diverse indole-substituted indanones via a rhodium(II)-catalyzed tandem reaction of N-sulfonyltriazoles with indoles was developed. The reaction involves rhodium(II)-catalyzed denitrogenation of the N-sulfonyltriazoles to form an oxonium ylide, followed by nucleophilic addition of the indoles and subsequent skeletal rearrangement. This strategy provides straightforward access to indanone frameworks bearing quaternary carbon centers.

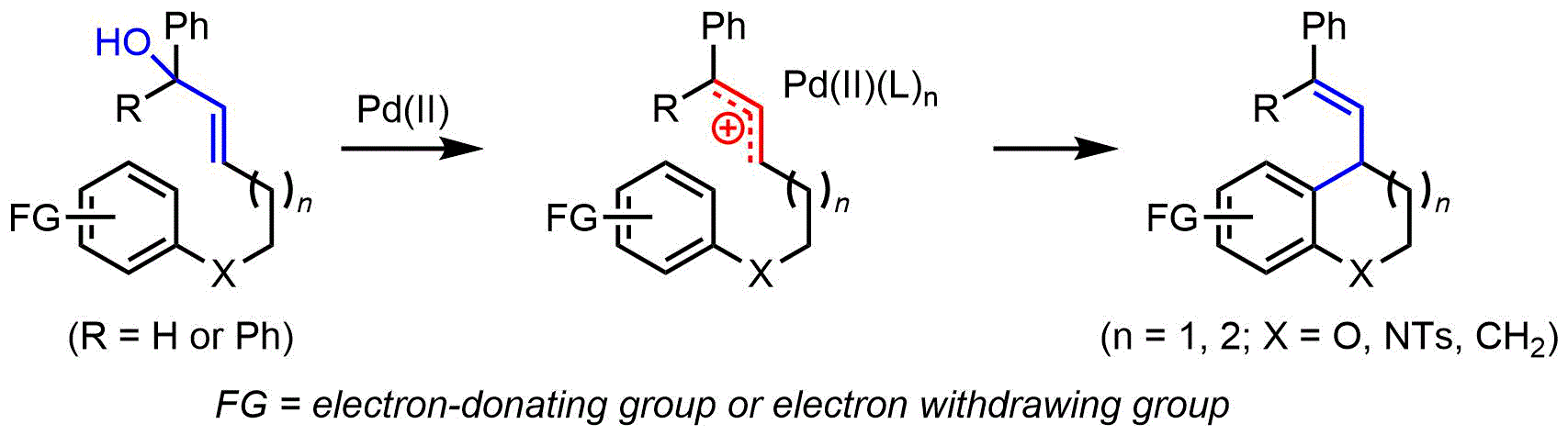
116.Synthesis of Substituted Benzoxacycles via a Pd(II)-Catalyzed Intramolecular Arylation Reaction of Allylic Alcohols
Jingjie Li, Ceheng Tan, Xinpeng Mu, Jianxian Gong*, Zhen Yang*
Herein a Pd-catalyzed intramolecular allylation reaction of unprotected allylic alcohols was developed, and the reaction proceeded through a Pd(II)-mediated allylic carbocation species formation, followed by a Friedel-Crafts type annulation to afford functionalized chromanes.