Publications
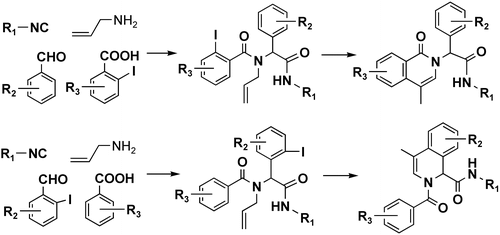
5.Concise Syntheisis of Isoquinoline via the Ugi and Heck Reactions
Z. Xiang, T. P. Luo, K. Lu, J. Y. Cui, X. Shi, R. Fathi, J. H. Chen*, Z. Yang*
Two types of isoquinoline scaffolds were successfully constructed in a combinatorial format via the Ugi four-component reaction and the Pd-catalyzed intramolecular Heck reaction, starting from readily available starting materials.
4.Synthesis of a Novel C2-Symmetric Thiourea and Its Application in the Pd-Catalyzed Cross-Coupling Reactions with Arenediazonium Salts under Aerobic Conditions
M. J. Dai, B. Liang, C. H. Wang, J. H. Chen*, Z. Yang*
A novel thiourea-based C2-symmetric ligand was synthesized, and its application in the palladium-catalyzed Heck and Suzuki coupling reactions of arenediazonium salts was evaluated. The reactions, which were performed at room temperature, without added base, and under aerobic conditions, produced product in 4 h with good yield. The corresponding arenediazonium salts were easily generated in one step from anilines.

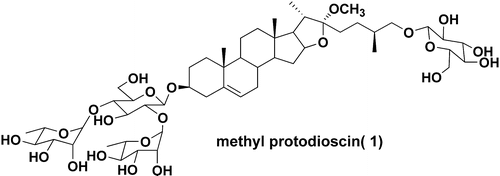
3.Total Synthesis of Methyl Protodioscin: A Potent Agent with Anti-tumor Activity
M. S. Cheng, Q. L. Wang, Q. Tian, H. Y. Song, Y. X. Liu, Q. Li, X. Xu, H. D. Miao, X. S. Yao*, Z. Yang*
Methyl protodioscin (1), otherwise known as 3-O-[α-l-rhamnopyranosyl-(1→2)-{α-l-rhamnopyranosyl-(1→4)}-β-d-glucopyranosyl]-26-O-[β-d-glucopyranosyl]-22-methoxy-25(R)-furost-5-ene-3β,26-diol, has been synthesized for the first time from diosgenin through nine steps in an overall yield of 7.8%.
2.Development of Thiourea-Based Ligands for the Palladium-Catalyzed Bis(methoxycarbonylation) of Terminal Olefins
M. J. Dai, C. H. Wang, J. Xiang, B. Liang, T. P. Luo, J. H. Chen*, Z. Yang*
Eur. J. Org. Chem. 2003, 4346.
Thiourea-based ligands were evaluated for the palladium-catalyzed bis(methoxycarbonylation) of terminal olefins. The usefulness of these ligands for this reaction is demonstrated by their stability to oxidizing agents, and their superiority in preventing palladium precipitation and double-bond isomerization. (© Wiley-VCH Verlag GmbH & Co. KGaA, 69451 Weinheim, Germany, 2003)

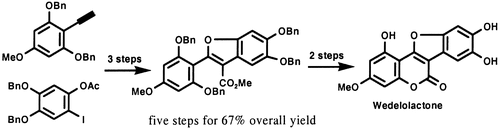
1.Total Synthesis of Wedelolactone
C. C. Li, Z. X. Xie, Y. D. Zhang, J.H.Chen*, Z. Yang*
The total synthesis of wedelolactone, a naturally occurring direct inhibitor of IKK complex that can suppress LPS-induced caspase-11 expression, using a convergent synthetic approach, is described. The key steps involved in this synthesis include the palladium-catalyzed Sonogashira reaction and the palladium-catalyzed carbonylative annulation reaction. This approach allows access to diversified analogues of wedelolactone.