Publications
30. Steteoselective Construction of an Unprecedented 7-8 Fused Ring System in Micrandilactone A by [3,3]-Sigmatropic Rearrangement
Y. D. Zhang, W. Ren, Y. Lan, Q. Xiao, K. Wang, J. Xu, J. H. Chen*, Z. Yang*
The 7−8 bicyclic ring system of micrandilactone A (1) with the required stereochemistry and functional groups was constructed by a Bu3Al-promoted Claisen rearrangement. Computational studies indicated that the exocyclic vinyl ether undergoes a [3,3] sigmatropic process via a chairlike transition state to afford exclusively the Z-double bond in the newly generated 8-membered ring with a high level of chirality transfer.

29.Effective Pd-Nanoparticle (PdNP)-Catalyzed Negishi Coupling Involving Alkylzinc Reagents at Room Temperature
J. Liu, Y. Deng, H. Wang, H. Zhang, G. Yu, B. Wu, H. Zhang, Q. Li, T. B. Marder*, Z. Yang*, A. W. Lei*
Pd(OAc)2 is an efficient catalyst precursor for Negishi coupling in the presence of Bu4NBr. Secondary and primary alkylzinc reagents with β-H and arylzinc reagents all reacted with aryl iodides at temperatures as low as −20 °C, giving moderate to good yields. One example of coupling between alkynylzinc reagents and aryl iodides was tested and the yield was good. Preliminary kinetic studies indicated that the process involved PdNPs as the active catalytic species.

28.A Concise and Diversity-Oriented Approach to the Synthesis of SAG Derivatives
N. D. Wang, J. Xiang, Z. Ma, J. M. Quan, J. H. Chen*, Z. Yang*
An efficient and rapid solution-phase combinatorial synthesis of the SAG library was developed. The salient features for this library synthesis is the application of carbothioamide-derived palladacycle-catalyzed Suzuki coupling reactions for the parallel synthesis of a series of pyridine-based biaryl aldehydes under aerobic conditions and a directN-alkylation of carbamates using NaH as base in DMF in the presence of catalytic amount of water. The resultant library has been submitted to biological screening to evaluate their potential role in the regulation of Hedgehog pathway.

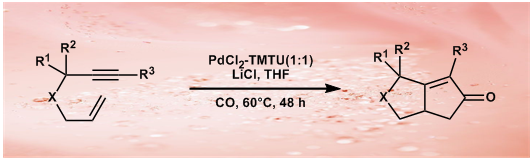
27.Effect of LiCl on Tuning the Reactivity of Pd-TMTU Catalyzed Pauson-Khand Reactions
L. J. Deng, J. Liu, J. Q. Huang, Y. Hu, M. Chen, Y. Lan, J. H. Chen*, A. Lei*, Z. Yang*
A general and robust transition-metal-catalyzed Pauson-Khand reaction is still difficult to achieve. In this contribution, we describe our recent observations on the effect of lithium chloride on Pauson-Khand reactions catalyzed by palladium(II) chloride-tetramethylthiourea; this allows structurally diverse cyclopentenones to be prepared effectively.
26.Synthesis of Thiourea-Oxazolines, a New Class of Chiral S,N-Heterobidentate Ligands: Application in Pd-Catalyzed Asymmetric Bis-methoxycarbonylation of Terminal Olefins
B. Liang, J. Liu, Y. X. Gao, D. X. Shu, K. Wongkhan, Y. Lan, A. Li, A. S. Batsanov, J. A. H. Howard, T. B. Marder*, J. H. Chen*, Z. Yang*
Organometallics, 2007, 26, 4756
A new chiral S,N-heterobidentate thiourea−oxazoline ligand was synthesized and isolated as two atropoisomers (4a, 4b). The ligands were employed in Pd-catalyzed enantioselective bis(alkoxycarbonylation)s of terminal olefins under mild conditions, giving high yields and modest ee values, demonstrating the potential of such ligands for use in Pd-catalyzed carbonylative reactions. Molecular structures of 4a and of the PdCl2 complexes of 4a and 4bhave been determined by single-crystal X-ray diffraction. In both complexes, the ligands exhibit a bidentate S,N bonding mode.





