Publications
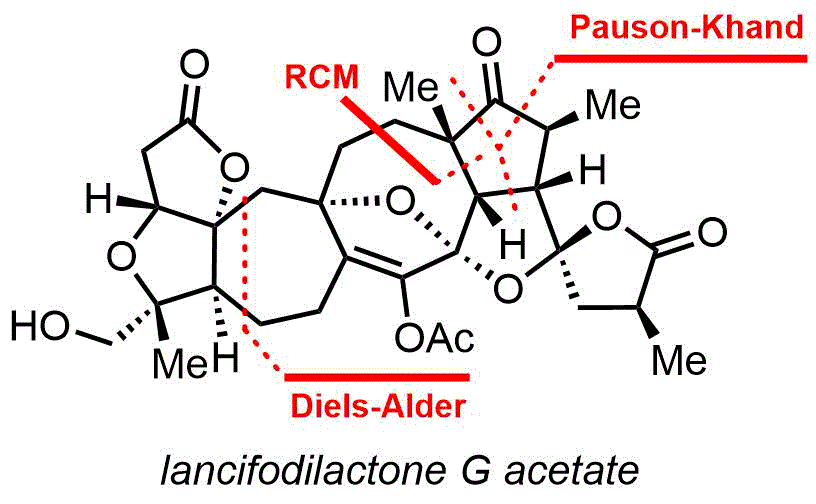
112. Asymmetric Total Synthesis of Lancifodilactone G Acetate
Dong-Dong Liu, Tian-Wen Sun, Kuang-Yu Wang, Yong Lu, Su-Lei Zhang, Yuan-He Li, Yan-Long Jiang, Jia-Hua Chen*, and Zhen Yang*
J. Am. Chem. Soc., 2017, 139, 5732–5735
Asymmetric total synthesis of structurally intriguing and highly oxygenated lancifodilactone G acetate (7) has been achieved for the first time in 28 steps from a cheap commodity chemical, 2-(triisopropylsiloxy)-1,3-butadiene.
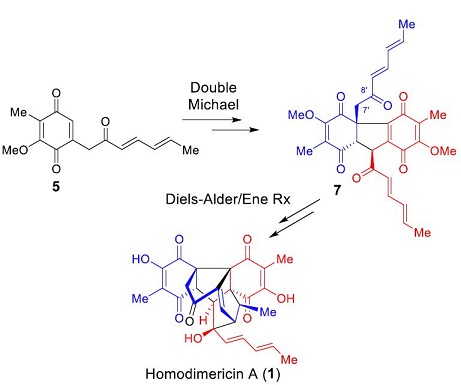
111. Bioinspired Total Synthesis of Homodimericin A
Jun Huang, Yueqing Gu, Kai Guo, Lei Zhu, Yu Lan*, Jianxian Gong* and Zhen Yang*
Angew. Chem. Int. Ed. 2017, 56, 7890
• Highlighted in Synfacts, 2017, 13, 906.
Homodimericin A is a remarkable fungal metabolite, and a highly oxygenated and racemic unsaturated polyketide. It poses a significant synthetic challenge due to its sterically demanding central cage-like core bearing eight contiguous stereogenic centers (including three contiguous all-carbon quaternary stereocenters), and several carbonyl functionalities. Based on its proposed biogenetic synthesis, we designed a bioinspired total synthesis of homodimericin A that proceeds in seven steps, featuring a double Michael reaction, an intramolecular Diels-Alder reaction, and an ene reaction.
110.Bioinspired Asymmetric Synthesis of Hispidanin A
Fuzhuo Li, Qian Tu, Sijia Chen, Lei Zhu, Yu Lan,* Jianxian Gong,* and Zhen Yang*
Angew.Chem. Int. Ed. 2017, 56,5844
• Selected as Hot Paper in Natural Products
• Highlighted in Synfacts, 2017, 13, 569.
The first enantiospecific synthesis of hispidanin A (4), a dimeric diterpenoid from the rhizomes of Isodon hispida, was achieved with a longest linear sequence of 12 steps in 6.5 % overall yield. A key component is the use of the abundant and naturally occurring diterpenoids (+)-sclareolide and (+)-sclareol as starting materials, which enables the gram-scale preparation of the key intermediates totarane (1) and s-trans-12E,14-labdadien-20,8β-olide (2). Subsequently a thermal or an erbium-catalyzed intermolecular Diels–Alder reaction of totarane (1) with labdadienolide (2) provide convergent and rapid access to the natural product hispidanin A (4). The synthetic studies have offered significant impetus for the efficient construction of these architecturally complex natural products.

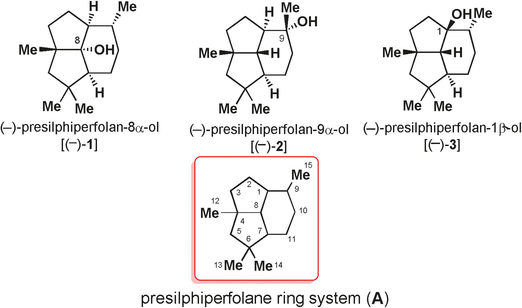
109. A Concise Synthesis of Presilphiperfolane Core through a Tandem TMTU–Co-Catalyzed Pauson–Khand Reaction and a 6π Electrocyclization Reaction
Zichun Zhang, Yuanhe Li, Dandan Zhao, Yingdong He, Dr. Jianxian Gong*, Prof. Dr. Zhen Yang*
The synthesis of strained polycyclic systems from readily available precursors with a minimum number of steps and with regio- and stereochemical control constitutes an important synthetic challenge. Herein, we report a tandem reaction comprising Co–TMTU (tetramethyl thiourea)-catalyzed Pauson–Khand (PK) and 6π-electrocyclization reactions for the formation of the highly strained core of presilphiperfolanols. The developed chemistry has been applied to the total syntheses of 4-epi-presilphiperfolan-8-ol and 7-epi-presilphiperfolan-1-ol.
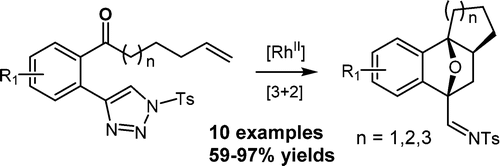
108.Stereoselective Synthesis of Oxabicyclo[2.2.1]heptenes via a Tandem Dirhodium(II)-Catalyzed Triazole Denitrogenation and [3 + 2] Cycloaddition
Hao Yuan, Jianxian Gong*, and Zhen Yang*
Org. Lett., 2016, 18 (21), 5500–5503
A novel synthetic strategy for the diastereoselective synthesis of structurally diverse oxabicyclo[2.2.1]heptenes has been developed, featuring a tandem reaction combining a Rh-catalyzed triazole denitrogenation and a novel type of [3 + 2] cycloaddition reaction. This tandem reaction was thought to proceed via a five-membered oxonium ylide intermediate, which was formed by the intramolecular nucleophilic attack of the carbonyl group on the α-imino metallocarbene followed by an inter- or intramolecular [3 + 2] dipolar cycloaddition with a range of alkynes and alkenes.