Publications
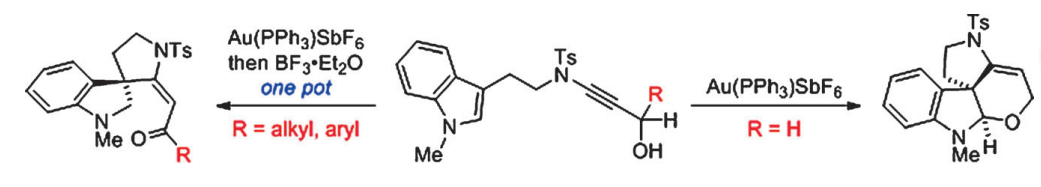
97.Gold-Catalyzed Intramolecular Tandem Cyclization of Indole-Ynamides: Diastereoselective Synthesis of Spirocyclic Pyrrolidinoindolines
Nan Zheng, Yuan-Yuan Chang, Li-Jie Zhang, Jian-Xian Gong,* and Zhen Yang*
A gold-catalyzed intramolecular tandem cyclization of indole-ynamide affords tetracyclic spirocyclic pyrrolidinoindoline bearing an all-carbon quaternary stereocentre in a single step; however, when the reaction was carried out in the presence of BF3⋅Et2O, the corresponding tricyclic spirocyclic pyrrolidinoindoline-based enones are produced through a key 1,5-hydride shift. The developed chemistry provides a diastereoselective and straightforward entry to structurally diverse polycylic pyrrolidinoindolines from indole-ynamides in one-pot reactions under mild conditions.
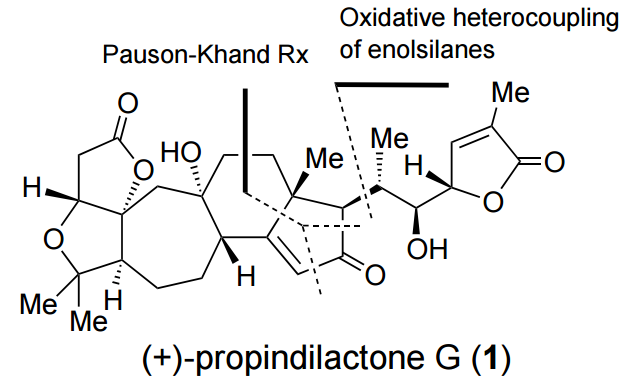
96. Asymmetric Total Synthesis of Propindilactone
Lin You, Xin-Ting Liang, Lingmin Xu, Yue-Fan Wang, Jia-Jun Zhang, Qi Su, Yuanhe Li, Bo Zhang, Shou-Liang Yang, Jia-Hua Chen*, and Zhen Yang*
J. Am. Chem. Soc., 2015, 137, 10120.
• Highlighted in Synfacts, 2015, 11, 1022.
95. Asymmetric Total Synthesis of (−)-Maoecrystal V
Wei-bin Zhang, Wen-bin Shao, Fu-zhuo Li, Dr. Jian-xian Gong*, Prof. Dr. Zhen Yang*
Chem. Asian J. 2015, 10, 1874.
• Highlighted in Org. Chem. Highlights 2016, August 22.
The asymmetric total synthesis of (−)-maoecrystal V, a novel cytotoxic pentacyclic ent-kaurane diterpene, has been accomplished. Key steps of the current strategy involve an early-stage semipinacol rearrangement reaction for the construction of the C10 quaternary stereocenter, a rhodium-catalyzed intramolecular O−H insertion reaction, and a sequential Wessely oxidative dearomatization/intramolecular Diels–Alder reaction to forge the pentacyclic framework of maoecrystal V.
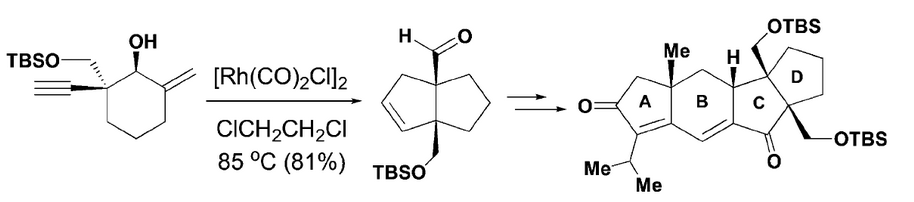
94.Diastereoselective Synthesis of Cyclopentanoids: Applications to the Construction of the ABCD Tetracyclic Core of Retigeranic Acid A [Inside Back Cover]
Junlin Zhang, Xiao Wang, Shuang Li, Dian Li, Song Liu, Prof. Dr. Yu Lan*, Dr. Jianxian Gong*, Prof. Dr. Zhen Yang*
A concise and efficient approach for the construction of the tetracyclic carbon skeleton of retigeranic acid A is described. The key transformations include a novel Rh-catalyzed [3+2] cycloaddition of enyol to afford cyclopentanoid E, bearing two contiguous quaternary stereocenters at the bridgehead positions, and an intramolecular Pauson–Khand reaction to construct the advanced tetracyclic core structure of retigeranic acid A.
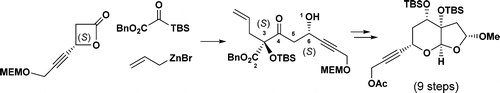
93. Synthetic Progress toward Azadirachtins. 2. Enantio- and Diastereoselective Synthesis of the Right-Wing Fragment of 11-epi-Azadirachtin I
Ceheng Tan, Wei Chen, Xinpeng Mu, Qi Chen, Jianxian Gong *, Tuoping Luo *, and Zhen Yang
A stereoselective three-component coupling reaction of allylzinc bromide, silyl glyoxylate, and a β-lactone has been developed. This has been successfully applied to the enantio- and diastereoselective synthesis of the fully functionalized furopyran moiety of azadirachtins.