Publications
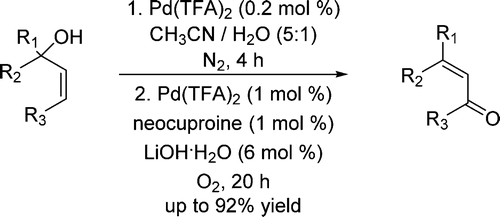
85.Palladium-Catalyzed Oxidative Rearrangement of Tertiary Allylic Alcohols to Enones with Oxygen in Aqueous Solvent
Jingjie Li , Ceheng Tan , Jianxian Gong *, and Zhen Yang *
A one-pot procedure for Pd(TFA)2-catalyzed 1,3-isomerization of tertiary allylic alcohols to secondary allylic alcohols followed by a Pd(TFA)2/neocuproine-catalyzed oxidative reaction to β-disubstituted-α,β-unsaturated kenones was developed.
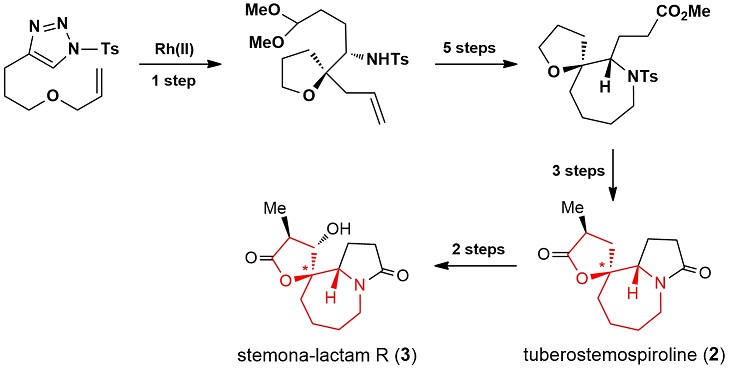
84.Concise Stereoselective Synthesis of Oxaspirocycles with 1-Tosyl-1,2,3-triazoles: Application to the Total Syntheses of (±)-Tuberostemospiroline and (±)-Stemona-lactam R
Junkai Fu, Hongjuan Shen, Yuanyuan Chang, Prof. Dr. Chuangchuang Li, Dr. Jianxian Gong and Prof. Dr. Zhen Yang*
• Highlighted in Org. Chem. Highlights, May, 11th, 2015.
A 4-substituted-1-tosyl-1,2,3-triazole-based stereoselective synthesis of structurally diverse oxaspirocycles is reported. The synthesis involves Rh-catalyzed loss of nitrogen from 4-substituted-1-tosyl-1,2,3-triazoles, Grignard reaction, and a ring-closing metathesis reaction as key steps. By employing readily available and stable 4-substituted-1-tosyl-1,2,3-triazoles as surrogates of diazo compounds and nitrogen sources, two types of oxaspirocycles were obtained. The latter compounds, which contain adjacent nitrogen stereocenters, could serve as the core structures of many natural products. This chemistry has been successfully applied to the total syntheses of (±)-tuberostemospiroline and (±)-stemona-lactam R.
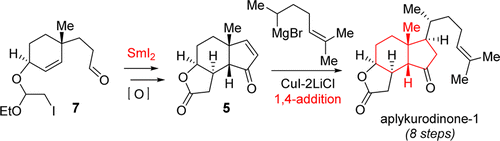
83.Total Synthesis of Aplykurodinone-1
Gang Liu, Guangjian Mei, Runwen Chen, Haina Yuan, Zhen Yang*, and Chuang-chuang Li*
The concise total synthesis of aplykurodinone-1 with an unusual cis-fused hydrindane moiety has been accomplished without the need for any protecting group chemistry using a unique SmI2 mediated reductive cascade cyclization reaction and a direct cuprate mediated 1,4-addition. This work represents the first example of the use of a SmI2-mediated intramolecular cascade cyclization reaction between “halide, alkene and aldehyde” groups.
82.Efficient Total Synthesis of Bioactive Natural Products: A Personal Record
Yun Zhang, Jianxian Gong,* and Zhen Yang*
In this account, we have highlighted our most recent works towards the total synthesis of bioactive natural products, which have resulted in the development of several novel synthetic methods. Inspired and guided by strategies based on diversity-oriented synthesis, we have successfully applied the novel synthetic methodologies developed in our lab to the total synthesis of a diverse collection of structurally challenging targets. We have also documented the evolution of these synthetic strategies. The total syntheses described in this account have been organized from the perspective of different molecules whilst still alluding to the parallel synthetic strategies involved.
81.Thioureas as Ligands in Organometallic Reactions
Jingjie Li, Li-Li Shi, Jiahua Chen, Jianxian Gong, Zhen Yang*
Thioureas are air and moisture stable, and they can coordinate to various metal centers, making their derivatives versatile ligands for transition-metal-catalyzed reactions. This account provides an overview of recent developments in the use of thioureas as ligands in organometallic-catalyzed reactions, with particular emphasis on their application to the total synthesis of natural products.