Publications
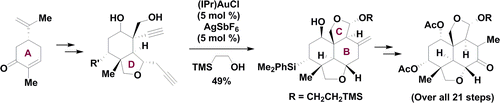
92. Synthetic Progress toward Azadirachtins. 1. Enantio- and Diastereoselective Synthesis of the Left-Wing Fragment of 11-epi-Azadirachtin I
Hang Shi, Ceheng Tan, Weibin Zhang, Zichun Zhang, Rong Long, Tuoping Luo *, and Zhen Yang *
A highly enantio- and diastereoselective synthesis of the left-wing fragment of 11-epi-azadirachtin I characterized with the pairwise use of palladium- and gold-catalyzed cascade reactions is presented. By enlisting a sequence of stereocontrolled transformations, our 21-step route established the stereocenters of the left-wing fragment from one chiral starting material, (−)-carvone, which would significantly facilitate the synthetic studies of the azadirachtin-type limonoids.
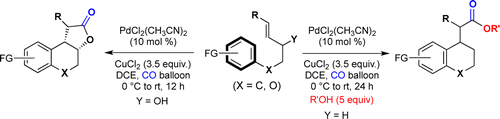
91.Palladium-Catalyzed Carbonylative Cyclization of Aryl Alkenes/Alkenols: A New Reaction Mode for the Synthesis of Electron-Rich Chromanes
Shuang Li, Fuzhuo Li, Jianxian Gong*, and Zhen Yang*
The Pd(II)-catalyzed intramolecular carbonylative cyclization reaction of aryl alkenes and aryl alkenols is reported for the synthesis of structurally diverse chromanes. PdCl2(CH3CN)2 was used as the catalyst and CuCl2 as the oxidant under the balloon pressure of CO. The reaction is conducted under mild conditions, and chromane-type esters and lactones can be generated in a highly regio- and stereoselective manner.
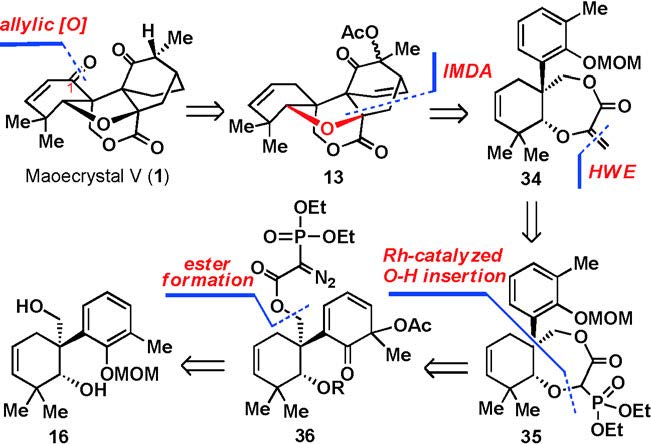
90.Total Synthesis of Maoecrystal V
Wei-Bin Zhang, Dr. Guang Lin, Wen-Bin Shao, Dr. Jian-Xian Gong,* and Prof. Dr. Zhen Yang*
Maoecrystal V (1) is a novel diterpenoid, which was originally isolated from the leaves of the Chinese medicinal herb Isodon eriocalyx in 2004 by Sun et al.1 It has been found to be selectively cytotoxic towards HeLa cells, with an IC50 value of 20 ng mL−1. Significant research efforts have been devoted to the synthesis of maoecrystal V because of its intriguing biological properties, rarity in nature, and complex structural features. Herein, we describe our recent investigations, which have culminated in the total synthesis of (±)-maoecrystal V. The current strategy involved three key steps for the successful construction of the key tetrahydrofuran oxa-bridge skeleton, including a Wessely oxidative dearomatization, a novel intramolecular Diels–Alder reaction, and a RhII-catalyzed O[BOND]H insertion reaction.

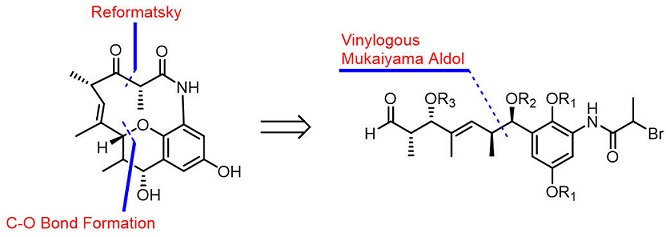
89. Asymmetric Total Synthesis of (−)-Cebulactam A1
Shouliang Yang, Yumeng Xi, Jia-Hua Chen* and Zhen Yang*
Org. Chem. Front., 2014, 1, 91-99
The total synthesis of (−)-cebulactam A1 (3) has been achieved for the first time in 18 steps. The key steps in this synthesis included an asymmetric chelation-controlled vinylogous Mukaiyama aldol reaction for the stereoselective synthesis of the stereogenic centers at the C8 and C9 positions, an intramolecular SmI2-mediated Reformatsky reaction for the formation of a macrocyclic lactam, and an SN2′ reaction for the stereoselective formation of the (E)-double bond linked tetrahydropyran moiety of cebulactam A1 (3).
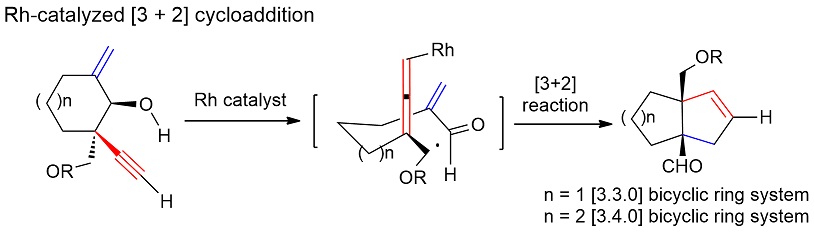
88.Asymmetric total synthesis of (−)-lingzhiol via a Rh-catalysed [3+2] cycloaddition
Rong Long, Jun Huang, Wenbin Shao, Song Liu, Yu Lan*, Jianxian Gong*, Zhen Yang*
The development of efficient reactions for the one-pot construction of bicyclic ring systems bearing two quaternary carbon centres at their bridgehead positions represents a significant challenge to synthetic chemistry. The development of new methods capable of overcoming this challenge is highly desirable, because this motif can be found in a wide range of natural products with significant biological activities. Herein, we report an efficient [3+2] cycloaddition reaction between an enal and an alleno rhodium species, which was generated in situ from the corresponding enynol via a retro metal-propargylation reaction, to give [3.3.0] and [3.4.0] bicyclic systems bearing two quaternary atoms at their bridgehead positions. The developed chemistry has been successfully applied to the asymmetric total synthesis of natural product (−)-lingzhiol (4) for the first time in 17 steps.