Publications
42.On the Mechanism of the Palladium Catalyzed Intramolecular Pauson-Khand-Type Reaction
Y. Lan, L. J. Deng, J. Liu, C. Wang, O. Wiest*, Z. Yang*, Y. D. Wu*
Density functional theory calculations and experimental studies have been carried out on the intramolecular Pauson−Khand-Type reaction mediated by a PdCl2-thiourea catalyst, which proceeds under mild reaction conditions and provides a useful alternative to traditional Pauson−Khand reactions. The classical mechanism of the Pauson−Khand reaction involving the alkyne/alkene C−C bond formation as the key step has been found to be energetically unfavorable and is not in line with the experimental observations. A novel reaction mechanism has been proposed for the reaction. The first step involves the cis-halometalation of the alkyne, followed by sequential alkene and carbonyl insertion. The rate-determining fourth step is an intramolecular C−Cl oxidative addition, leading to a PdIV intermediate. A C−C bond formation by reductive elimination completes the reaction. The mechanism is in agreement with the key experimental observations including (1) the need of a chloride for catalytic activity and the absence of catalysis with Pd(OAc)2 alone; (2) the rate acceleration by the addition of LiCl; both with PdCl2 and Pd(OAc)2 catalysts; and (3) the preferred formation of the trans diastereomer in substituted cases. The cis halometalation and the formation and stability of the PdIV intermediate is studied in detail and provides general insights into these novel steps.

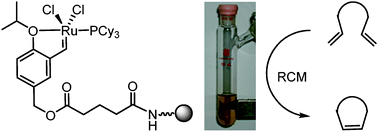
41.Magnetic nanoparticle-supported Hoveyda-Grubbs catalysts for ring-closing metathesis reactions
C. Che, W. Z. Li, S. Y. Lin, J. W. Chen, J. Zheng, J. C. Wu, Q. X. Zheng, G. Q. Zhang, Z. Yang* and B. W. Jiang*
Magnetically recyclable Hoveyda–Grubbs catalyst can be readily assembled using magneticnanoparticles as support, and this catalyst combines convenient recyclability and excellent activity on ring-closing metathesis (RCM) reactions.
*This publication was highlighted on http://www.organic-chemistry.org/Highlights/2010/28June.shtm
40.Synthetic Study toward the Total Synthesis of Maoecrystal V
J. X. Gong, G. Lin, C. C. Li* and Z. Yang*
A novel and concise approach for the construction of the core structure of maoecrystal V (1) has been developed. Utilizing the lead-mediated arylation of β-ketoesters and oxidative dearomatization/IMDA reaction as key steps, the two consecutive all-carbon quaternary centers (C-9 and C-10) were constructed in a stereoselective manner. The developed chemistry paves the way for the total synthesis of this fascinating natural product.

39. Unexpected Regioselectivity in the Synthesis of Pyranonaphthoquinone via the Diels−Alder Reaction
Y. Cui, H. Jiang, Z. T. Li, N. Wu, Z. Yang* and J. M. Quan*
The unusual regioselectivity in the Diels−Alder reactions of pyranoquinone 1 with (4,4-dimethoxybuta-1,3-dien-2-yloxy)trimethylsilane 2 are explored by both computations and experiments. The regioselectivity is controlled by the electrostatic interaction of the lactone ring-oxygen and the vicinal quinone oxygen on the transition structure, which can be tuned by the terminal methyl group of the butadienes.

38.Formal Total Synthesis of N-Methylmaysenine
L. Wang, J. X. Gong, L. J. Deng, Z. Xiang, Z. X. Chen, Y. Wang, J. H. Chen*, Z. Yang*
A novel synthetic approach for the formal total synthesis of N-methylmaysenine (1) has been developed. Key steps involve the Ti-mediated vinylogous Mukaiyama aldol reaction of chiral ketene silyl N,O-acetal with β-dithiane-substituted aldehyde, an aldol condensation, and a ring-closing metathesis reaction.