Publications
50.Asymmetric Total Synthesis of Caribenol A
L. Z. Liu, J. C. Han, G. Z. Yue, C. C. Li*, Z. Yang*
J. Am. Chem. Soc. 2010, 132, 13608
• Top5 Most Accessed Article in J. Am. Chem. Soc. of 2010.
• Highlighted in Nature Chemistry, September, 2010.
• Highlighted in Chin. J. Org. Chem., November, 2010.
• “Highlight syntheses” in Annu. Rep. Prog. Chem., Sect. B: Org. Chem., 2011.
• Highlighted in Org. Chem. Highlights, August, 2011.
A unified strategy toward the asymmetric total synthesis of carbenol A is reported, featuring intramolecular Diels−Alder (IMDA) and biomimetic oxidation reactions as key steps.

49.Benzo[e]isoindole-1,3-diones as Potential Inhibitors of Glycogen Synthase Kinase-3 (GSK-3). Synthesis, Kinase Inhibitory Activity, Zebrafish Phenotype, and Modeling of Binding Mode
H. X. Zou, L. Y. Zhou, Y. Z. Li, Y. Cui, H. B. Zhong, Z. Y. Pan, Z. Yang and J. M. Quan
Benzo[e]isoindole-1,3-dione derivatives were synthesized, and the effects on GSK-3β activity and zebrafish embryo growth were evaluated. A series of derivatives show obvious inhibitory activity against GSK-3β. The most potent inhibitor, 7,8-dimethoxy-5-methylbenzo[e]isoindole-1,3-dione (8a), shows nanomolar IC50 and obvious phenotype on zebrafish embryo growth associated with the inhibition of GSK-3β at low micromolar concentration. The interaction mode between 8a and GSK-3β was characterized by computational modeling.

48.Construction of All-Carbon Quaternary Center by R2AlCl−Mediated Ring-Opening Reaction of Oxacycles
C. Che, L. Z. Liu, J. X. Gong, Y. F. Yang, G. X.Wang, J. M. Quan* and Z. Yang*
An unexpected R2AlCl-mediated ring-opening reaction of oxacycles for the formation of all-carbon quaternary centers was discovered, and a possible mechanism is proposed. The developed chemistry provides a concise approach to synthesize structural diverse of dolastane-type compounds.

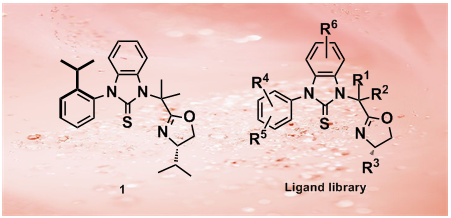
47.A Thiourea-Oxazoline Library with Axial Chirality: Ligand Synthesis and Studies of the Palladium-Catalyzed Enantioselective Bis(methoxycarbonylation) of Terminal Olefins
Y. X. Gao, L. Chang, H. Shi, B. Liang, K. Wongkhan, D. Chaiyaveij, A.S. Batsanov, T. B. Marder*, C. C. Li*, Z.n Yang*, Y. Huang*
Adv, Synth Catal. 2010, 352, 1955
We report herein the synthesis of novel chiral S,N-heterobidentate thiourea-oxazoline ligands and their application to palladium-catalyzed enantioselective bis(methoxycarbonylation)s of terminal olefins under mild conditions. Copper salts were found to play multiple roles in this reaction. Substituted 2-phenylsuccinates were obtained in >90% yield and up to 84% ee under optimized conditions.
46.Development of New Stereodiverse Diaminocyclitols as Inhibitors of Influenza Virus Neuraminidase
Y. Cui, Z. D. Jiao, J. X. Gong, Q. Yu, X. F. Zheng, J. M. Quan*, M. Luo* and Z. Yang*
A concise and modular approach to synthesize a new type of cyclopentene-based diaminocyclitol library from d-serine and l-serine has been developed, and key steps in this synthesis are an aza-Claisen rearrangement, a ring-closing metathesis, and a Baylis−Hillman reaction. The developed chemistry may offer a unique way to investigate the neuraminidase (NA) mutation by systematically mapping the changes within its binding sites.





