Publications
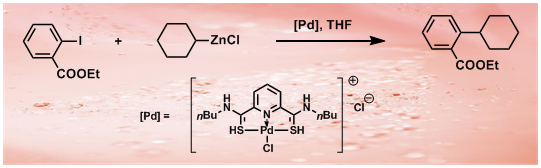
35. Pincer Thioamide and Pincer Thioimide Palladium Complexes Catalyze Highly Efficient Negishi Coupling of Primary and Secondary Alkyl Zinc Reagents at Room Temperature
H. B. Wang, J. Liu, Y. Deng, T. Y. Min, G. X. Yu, X. J. Xu, Z. Yang, A. W. Lei*
Chem. Eur. J. 2009, 15, 1499-1507.
Pincer thioamide PdII complex 2 was prepared, and its reaction with cyclohexylzinc chloride yielded novel pincer thioimide PdII complex 3 besides Pd0 species. The structures of complexes 2 and 3 were confirmed by X-ray analysis. Both complexes are efficient catalysts for Negishi couplings involving primary and secondary alkyl zinc reagents bearing β-hydrogen atoms. At a concentration of 0.1–0.5 mol % both catalysts readily promoted reactions at room temperature or even at 0 °C. The operational simplicity of these processes, in conjunction with the easy accessibility of both catalysts and substrates, promises synthetic utility of this new methodology. An experiment on a scale of 19.35 g carried out at very low catalyst loading of 2 (turnover number: 6 100 000) highlighted the potential application of the catalytic system. Monoalkyl and dialkyl zinc reagents displayed different reactivities and selectivities in reactions with aryl iodides catalyzed by complexes 2 or 3, and isomerization in reactions involving acyclic secondary alkyl zinc derivatives was suppressed by using appropriate amounts of dialkyl zinc reagents. Based on preliminary kinetic profiles and reaction evidence, three possible pathways are proposed for the reactions involving acyclic secondary alkyl zinc reagents to rationalize the difference between mono-alkyl and dialkyl zinc derivatives.
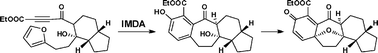
34.A model study for the concise construction of the oxapentacyclic core of cortistatins through intramolecular Diels-Alder and oxidative dearomatization-cyclization reactions
L. Z. Liu, Y. X. Gao, C. Che, N. Wu, D. Z. Wang, C. C. Li*, Z. Yang*
A unified strategy towards the facile construction of the [6.7.6.5] oxapentacyclic skeleton of cortistatins is reported, featuring intramolecular Diels–Alder (IMDA) and oxidative dearomatization–cyclization reactions as key steps.
33.Synthesis of Catechins via Thiourea/AuCl3-Catalyzed Cycloalkylation of Aryl Epoxides
Y. X. Liu, X. Li, G. Li, Z. Xiang, J. Xiang, M. Z. Zhao, J. Chen*, Z. Yang*
A diversity-oriented approach for the synthesis of structurally diverse catechins was achieved in good yields via thiourea/AuCl3/AgOTf-catalyzed annulations of aryl epoxides under mild conditions. This new protocol provides a highly efficient entry to a library of catechins-derived natural products, notably anti-HIV agent 8-C-ascorbyl-(−)-epigallocatechin.

32.Total Synthesis of Crisamicin A
Z. T. Li, Y. X. Gao, Y. F. Tang, M. J. Dai, G. X. Wang, Z. G. Wang* and Z. Yang*
Stereoselective total synthesis of natural product crisamicin A (1) was accomplished for the first time via the Pd/TMTU-catalyzed alkoxycarbonylative annulation to generate a uniquecis-pyran-fused lactone, an intermolecular Diels−Alder reaction to construct the pyranonaphthoquinone unit, and a novel Pd−thiourea pincer complex-catalyzed homocoupling of functionalized naphthoquinones.

31.Diversity-Oriented Synthesis of Fused Pyran v-Lactones via an Efficient Pd−Thiourea-Catalyzed Alkoxycarbonylative Annulation
Z. T. Li, Y. X. Gao, Z. D. Jiao, N. Wu, D. Z. G. Wang* and Z. Yang*
We reported herein a diversity-oriented synthesis of a range of fused pyran-γ-lactones that was effected through a versatile Pd−thiourea complex-catalyzed intramolecular alkoxycarbonylative annulation.