Publications
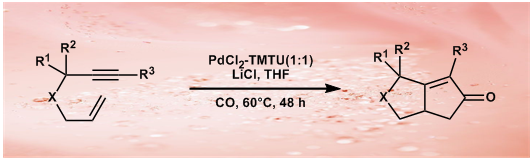
27.Effect of LiCl on Tuning the Reactivity of Pd-TMTU Catalyzed Pauson-Khand Reactions
L. J. Deng, J. Liu, J. Q. Huang, Y. Hu, M. Chen, Y. Lan, J. H. Chen*, A. Lei*, Z. Yang*
A general and robust transition-metal-catalyzed Pauson-Khand reaction is still difficult to achieve. In this contribution, we describe our recent observations on the effect of lithium chloride on Pauson-Khand reactions catalyzed by palladium(II) chloride-tetramethylthiourea; this allows structurally diverse cyclopentenones to be prepared effectively.
26.Synthesis of Thiourea-Oxazolines, a New Class of Chiral S,N-Heterobidentate Ligands: Application in Pd-Catalyzed Asymmetric Bis-methoxycarbonylation of Terminal Olefins
B. Liang, J. Liu, Y. X. Gao, D. X. Shu, K. Wongkhan, Y. Lan, A. Li, A. S. Batsanov, J. A. H. Howard, T. B. Marder*, J. H. Chen*, Z. Yang*
Organometallics, 2007, 26, 4756
A new chiral S,N-heterobidentate thiourea−oxazoline ligand was synthesized and isolated as two atropoisomers (4a, 4b). The ligands were employed in Pd-catalyzed enantioselective bis(alkoxycarbonylation)s of terminal olefins under mild conditions, giving high yields and modest ee values, demonstrating the potential of such ligands for use in Pd-catalyzed carbonylative reactions. Molecular structures of 4a and of the PdCl2 complexes of 4a and 4bhave been determined by single-crystal X-ray diffraction. In both complexes, the ligands exhibit a bidentate S,N bonding mode.

25.A One-Pot Synthesis of Quinoline-based Tetracycles by a Tandem Three-Component Reaction
C. Che, J. Xiang, G. X. Wang, R. Fathi, G. J. M. Quan, Z. Yang*
A practical one-pot synthetic strategy for the efficient synthesis of a range of structurally interesting and bioactive quinoline-based tetracycles has been developed. A key step in the synthesis is a tandem three-component reaction of heteroaromatic amine, methyl 2-formylbenzoate and tbutyl isonitrile, followed by TFA-mediated lactamization via intramolecular aminolysis of an adjacent ester. Results related to a kinase-panel screening for several selected compounds are also discussed in this article.

24.An Efficient One-Pot Asymmetric Synthesis of Biaryl Compounds via Diels-Alder/Retro-Diels-Alder Cascade Reactions
Y. X. Liu, K. Lu, M. J. Dai, K. Wang, W. Wu, J. H. Chen*, Z. Yang*
A single-step chirality transfer method for the synthesis of axially chiral biaryl compounds by construction of the second aromatic ring via a Diels−Alder/retro-Diels−Alder cascade reaction is reported. This methodology should find broad application in the synthesis of natural products and asymmetric catalysts.

*This publication was highlighted on http://www.organic-chemistry.org/Highlights/2007/08October.shtm
23.Efficient Synthesis of Maleimides and Carbazoles via Zn(OTf)2-Catalyzed Tandem Annulations of Isonitriles and Allenic Esters
Y. Z. Li, H. X. Zou, J. X. Gong, J. Xiang, T. P. Luo, J. M. Quan, G. X. Wang*, and Z. Yang*
Lewis acid Zn(OTf)2-catalyzed tandem annulations of isonitriles and allenic esters which lead to efficient and flexible syntheses of a range of biologically significant maleimides and carbazoles and related compounds are reported. A mechanistic rationale is proposed to account for the observed reactivity.





