Publications
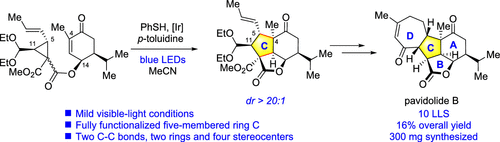
137.Asymmetric Total Synthesis of (−)-Pavidolide B via a Thiyl-Radical-Mediated [3 + 2] Annulation Reaction
Pengpeng Zhang, Yuanhe Li, Zhiming Yan, Jianxian Gong*, Zhen Yang*
J. Org. Chem. 2019, 84(24), 15958-15971
The development of an efficient strategy for the asymmetric total synthesis of the bioactive marine natural product (−)-pavidolide B is described in detail. The development process and detours leading to the key thiyl-radical-mediated [3 + 2] annulation reaction, which constructed the central C ring with four contiguous stereogenic centers in one step, are depicted. Subsequently, the seven-membered D ring is constructed via a ring-closing metathesis reaction followed by a Rh(III)-catalyzed isomerization. This strategy enables the total synthesis of (−)-pavidolide B in the longest linear sequence of 10 steps.
136.Diastereoselective Construction of All-Carbon Quaternary Stereocenters via Intramolecular Oxidative Cross-Coupling Reaction
Wei Chen, Renyu Guo, Jianxian Gong*, Zhen Yang*
Chin. J. Org. Chem. 2019, 39 (1), 238-248
The formation of sterically hindered C—C bond represents a great challenge in modern synthetic organic chemistry. A particularly challenging issue is the construction of all-carbon quaternary stereocenters. Herein, a ceric ammonium nitrate (CAN)-mediated intramolecular oxidative cross-coupling of silyl ethers for direct construction of valuable polycyclic scaffolds is described. The reaction enables sterically congested vicinal all-carbon quaternary and tertiary stereocenters to be installed diastereoselectively. The developed method provides a concise and efficient approach for ligation of two different segments through a compact C—C bond formation, which has potential applications in the synthesis of com plex molecules as well as sterically congested natural products.
135.Concise synthesis of the core structure of madreporanone by Rh-catalyzed [3+2] cycloaddition
Rong Long, Zhen Yang*
Tetrahedron 2019, 75 (12), 1746-1750
A model study toward total synthesis of madreporanone, a novel diterpene isolated from Azorella madreporica, was investigated. The [3.3.0] bicyclic core of madreporanone bearing a cis-configured isopropyl group and two vicinal quaternary carbon centers was stereoselectively constructed via an intramolecular Rh-catalyzed [3 + 2] cycloaddition in a single step.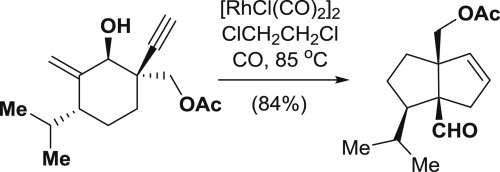
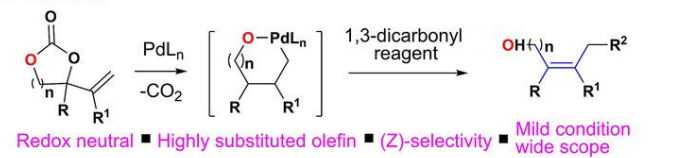
134.Pd-Catalyzed Decarboxylative Allylation for Stereoselective Syntheses of Allylic Alcohols bearing a Quaternary Carbon Center
Linlin Shi, Yingdong He, Jianxian Gong*, Zhen Yang*
Asian J. Org. Chem 2019, 8 (6), 823-827
Olefins play a vital role in the fields of bioscience, medicine, and chemistry. Stereo‐defined olefins are important resource in both chemical laboratory and industry. Using a palladium‐catalyzed decarboxylative transformation of vinyl cyclic carbonates, we have developed an efficient and reliable method for the direct construction of highly substituted olefins with the concomitant formation of a new Csp3‐Csp3 bond. The desired olefins could be generated in reasonable yields with good stereoselectivities, in contrast to the existing methods, which are usually non‐stereoselective. The palladium‐catalyzed decarboxylative transformation will undoubtedly provide new synthetic routes for the formation of multi‐substituted allylic scaffolds.
133.Total syntheses of dehydrobotrydienal, dehydrobotrydienol and 10-oxodehydrodihydrobotrydial
Zichun Zhang, Dandan Zhao, Yingdong He, Zhen Yang, Jianxian Gong
Chin. Chem. Lett. 2019, 30 (8), 1503-1505
In this paper, we report the concise total syntheses of three botryane sesquiterpenoids: dehydrobotrydienal, dehydrobotrydienol, and 10-oxodehydrodihydrobotrydial. The key transformations include tandem Co-tetramethylthiourea-catalyzed Pauson–Khand and 6π-electrocyclization reactions to forge the tricyclic core structure of the botryanes, and further oxidative aromatization and oxidation to complete the total syntheses.