Publications
145.Asymmetric Total Synthesis of (−)-Spirochensilide A
Xin-Ting Liang, Jia-Hua Chen,* and Zhen Yang*
J. Am. Chem. Soc. 2020, 142(18), 8116–8121
An asymmetric total synthesis of (−)-spirochensilide A has been achieved for the first time. The synthesis features a semipinacol rearrangement reaction to stereoselectively construct the two-vicinal quaternary chiral centers at C8 and C10, a tungsten-mediated cyclopropene-based Pauson–Khand reaction to install the C13 quaternary chiral center, and a furan-based oxidative cyclization to stereoselectively form the spiroketal motif.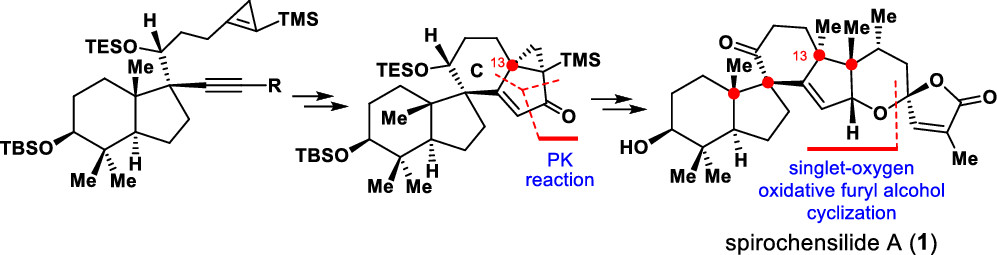
144.Photoredox‐Catalyzed Isomerization of Highly‐Substituted Allylic Alcohols via C‐H bond activation
Kai Guo, Zhongchao Zhang, Anding Li, Yuanhe Li, Jun Huang,* Zhen Yang,*
143.Asymmetric Total Synthesis of (+)-Waihoensene
Yongzheng Qu, Zheyuan Wang, Zhongchao Zhang, Wendou Zhang, Jun Huang,* and Zhen Yang*
J. Am. Chem. Soc. 2020, 142(14), 6511-6515
• Highlighted in ChemistryViews, 07 Apr 2020
The asymmetric total synthesis of (+)-waihoensene, which has a cis-fused [6,5,5,5] tetracyclic core bearing an angular triquinane, a cis-fused six-membered ring, and four contiguous quaternary carbon atoms, was achieved through a sequence of chemical reactions in a stereochemically well-defined manner. The total synthesis features the following: (1) Cu-catalyzed asymmetric conjugated 1,4-addition; (2) diastereoselective Conia-ene type reaction; (3) diastereoselective intramolecular Pauson–Khand reaction; (4) Ni-catalyzed diastereoselective conjugated 1,4-addition; and (5) radical-initiated intramolecular hydrogen atom transfer (HAT). Control experiments and density functional theory calculations support the proposed HAT process.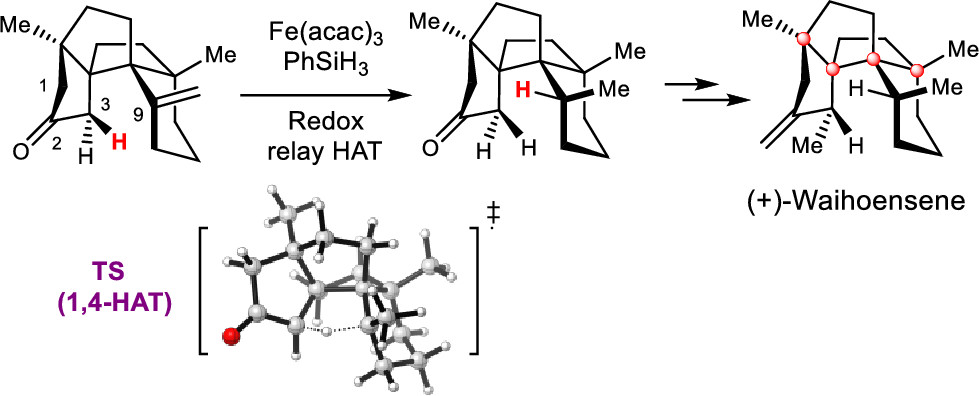
142.Asymmetric Total Synthesis of (−)-Guignardones A and B
Zhiming Yan, Chunbo Zhao, Jianxian Gong,* and Zhen Yang*
Org. Lett. 2020, 22(4), 1644-1647
The asymmetric total synthesis of (−)-guignardones A (2) and B (1) has been accomplished. The highly oxidized 6-oxabicyclo[3.2.1]octane core was constructed from d-quinic acid via substitution/desulfurization reaction with thiophenol to forge the bridged ring scaffold, and a Pummerer rearrangement and 1,4-addition/elimination sequence was employed to install the β-carbonyl group at the congested C-1 position. A late-stage Knoevenagel condensation–6π-electrocyclization and directed hydrogenation formed (−)-guignardone B (1), which was subjected to dehydration to furnish (−)-guignardone A (2).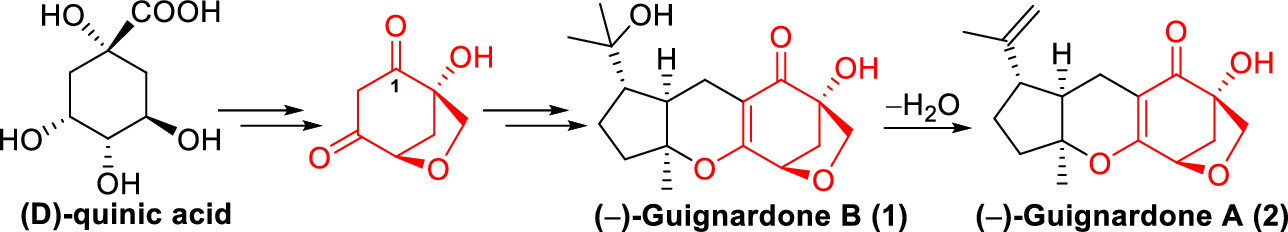
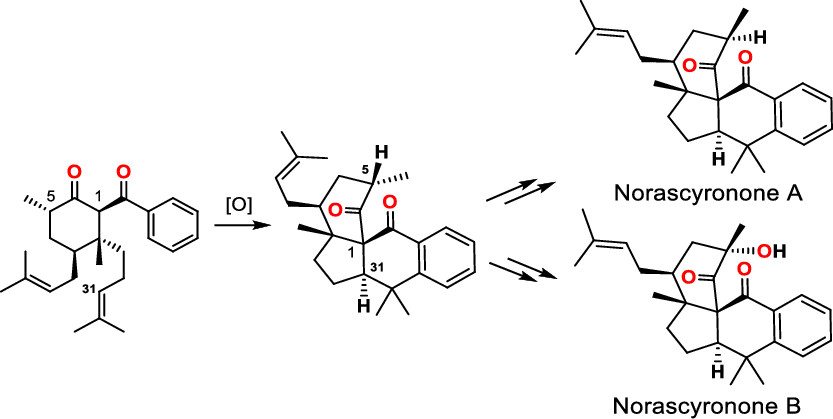
141.Protecting-Group-Free Total Syntheses of (±)-Norascyronones A and B
Tingting Cao, Lei Zhu, Yu Lan*, Jun Huang* and Zhen Yang*
Org. Lett. 2020, 22(7), 2517-2521
Protecting-group-free total syntheses of natural products norascyronone A and norascyronone B were accomplished in eight steps from the commercially available starting material 1-bromo-4-methoxy-2-methylbenzene. The key step was a Mn/Cu-mediated oxidative cascade annulation reaction that formed the tetracyclic core of the target molecules bearing vicinal bridge-head all-carbon quaternary chiral centers. Our investigation indicated that the C5 stereogenic center of norascyronone C plays a critical role in the proposed biomimetic oxidative reaction for B-ring formation.