Publications
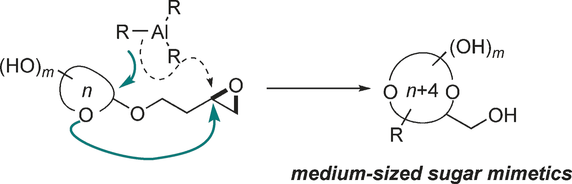
107.A Migratory Ether Formation Route to Medium-Sized Sugar Mimetics
, , , , ,* and *
Angew. Chem. Int. Ed. 2016, 55, 14340-14344
Polyol-substituted cyclic ethers are fundamental building blocks of biomolecules. The position and stereochemistry of multiple hydroxy substituents of cyclic ethers play a central role in their biological function. Current methods for the synthesis of such structures are limited to “naked” ring products with no or few substituents. Here we describe a general route to medium-sized polyol cyclic ethers using a migratory ether formation strategy. In contrast to the common pathway of direct opening of epoxides, Me3Al was found to promote an unprecedented ether addition reaction, opening a neighboring epoxide. The resulting oxonium intermediate triggers a 1,3-methyl shift to yield 2-deoxyribital products. When the hemiacetal auxiliary is a monosaccharide, the sugar ring is expanded by four atoms to give the corresponding 9- to 11-membered analogues. This method provides an entry into the untapped chemical space of medium-sized sugar mimetics.
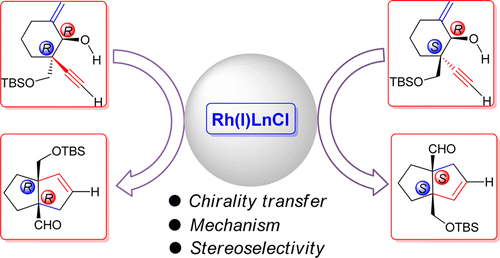
106.Effective Chirality Transfer in [3+2] Reaction between Allenyl-Rhodium and Enal: Mechanistic Study Based on DFT Calculations
Xiaotian Qi, Song Liu, Tao Zhang, Rong Long, Jun Huang, Jianxian Gong*, Zhen Yang*, and Yu Lan*
Theoretical calculation was performed to study the chirality transfer in a newly reported intramolecular [3+2] cycloaddition of enal and alleno rhodium species, generated in situ from an enynol precursor. [3.3.0] bicyclic system which contains two bridgehead quaternary carbons that can be achieved, the chirality of which are controlled by those of the starting material, and the product stereoselectivity is only determined by the α-position of the acetylene moiety. Density functional theory calculations predicted that only the cis [3.3.0] bicyclic product could be generated, regardless of either erythro or threo substrate, which was also confirmed by experimental observations.
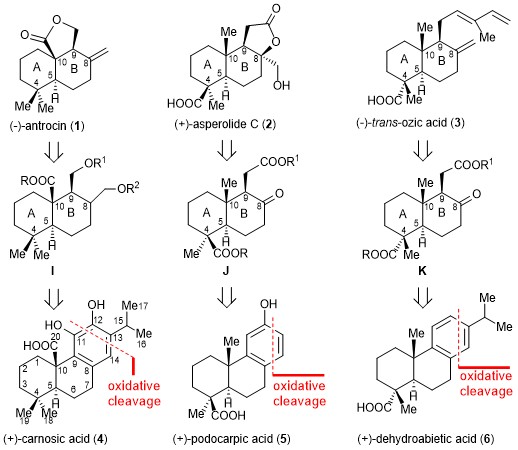
105.A chiral pool approach for asymmetric syntheses of (-)-antrocin, (+)-asperolide C, and (-)-trans-ozic acid
Fu-Zhuo Li, Shuang Li, Peng-Peng Zhang, Zhi-Hui Huang, Wei-Bin Zhang,* Jianxian Gong* and Zhen Yang*
Chem. Commun., 2016,52, 12426-12429
Ozonolysis of aromatic abietane (+)-carnosic acid (4) is used to create an important intermediate in an enantiomerically pure form, resulting in a simple, concise, readily scalable, and asymmetric synthesis of (−)-antrocin (1). This strategy not only provides an efficient approach to (−)-antrocin (1) synthesis but can also be readily adopted for the syntheses of optically pure (+)-asperolide C (2) and (−)-trans-ozic acid (3) from the naturally abundant aromatic abietanes (+)-podocarpic acid (5) and (+)-dehydroabietic acid (6). The strategy presented here is an example of the use of naturally occurring aromatic abietanes as a chiral pool and offers an account of the asymmetric synthesis of terpenoids.
104.Exploring the Complexity-Generating Features of the Pauson–Khand Reaction from a Synthetic Perspective
Lili Shi, Zhen Yang*
Eur. J. Org. Chem. 2016, 2356–2368
The Pauson–Khand reaction (PKR) is used to construct a cyclopentenone framework from an alkene, an alkyne, and carbon monoxide, through a domino sequence of bond constructions. This microreview covers the synthetic application of the PKR in the total synthesis of a collection of structurally challenging natural products in the authors’ laboratory over the past 10 years, beautifully exploring the complexity-generating features of the PKR. Key to the success of these total syntheses is the implementation of the PKR to access complex target molecules containing numerous stereocenters.
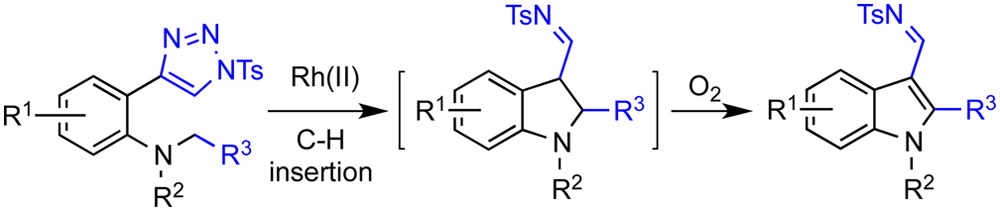
103.Synthesis of 2,3-Disubstituted Indoles and Benzofurans by the Tandem Reaction of Rhodium(II)-Catalyzed Intramolecular C–H Insertion and Oxygen-Mediated Oxidation
Hongjuan Shen, Junkai Fu, Hao Yuan, Jianxian Gong*, and Zhen Yang*
J. Org. Chem., 2016, 81, 10180–10192
A highly effective and straightforward method to construct a wide range of functionalized 2,3-disubstituted indoles has been developed. The method involves the tandem reaction of rhodium(II)-catalyzed denitrogenative annulation of triazole-based benzyl anilines and oxygen-mediated oxidative aromatization. The developed method can also be used to synthesize 2,3-disubstituted benzofurans by replacing the benzyl anilines with benzyl phenols.