Publications
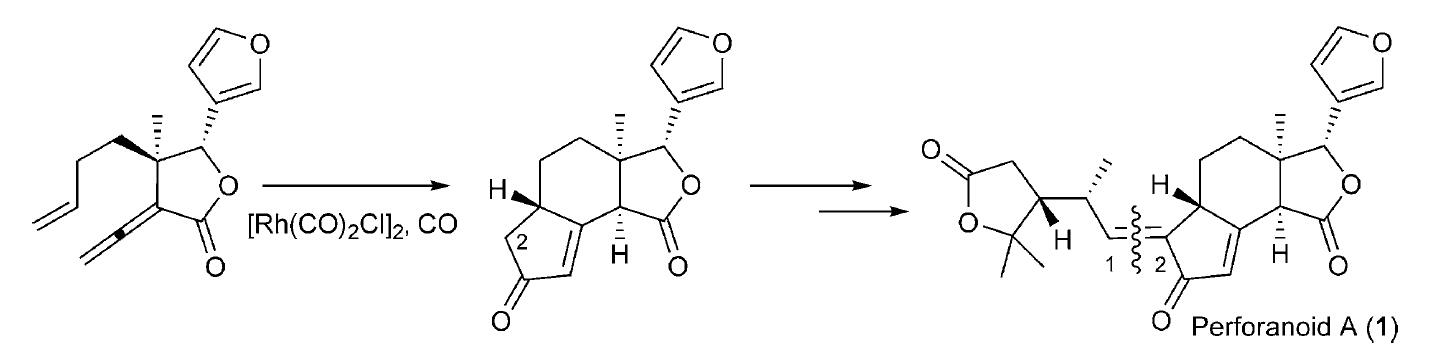
102.Isolation and Asymmetric Total Synthesis of Perforanoid A
Chao Lv , Xiaohui Yan , Qian Tu, Yingtong Di, Chunmao Yuan, Xin Fang, Yaacove Ben-David, Lei Xia, Jianxian Gong, Yuemao Shen,* Zhen Yang,* and Xiaojiang Hao*
Angew. Chem. Int. Ed. 2016, 55, 7539
• Highlighted in Org. Chem. Highlights 2017, March 6.
A novel limonoid, perforanoid A, was isolated, and an asymmetric total synthesis was achieved in 10 steps. The key steps are chiral tertiary aminonaphthol mediated enantioselective alkenylation of an aldehyde to an allylic alcohol, Pd-catalyzed coupling of the allylic alcohol with vinyl ether to form the γ-lactone ring, and cyclopentenone ring formation through a Rh-catalyzed Pauson–Khand reaction. Preliminary studies show that perforanoid A is cytotoxic towards HEL, K562, and CB3 tumor cell lines.
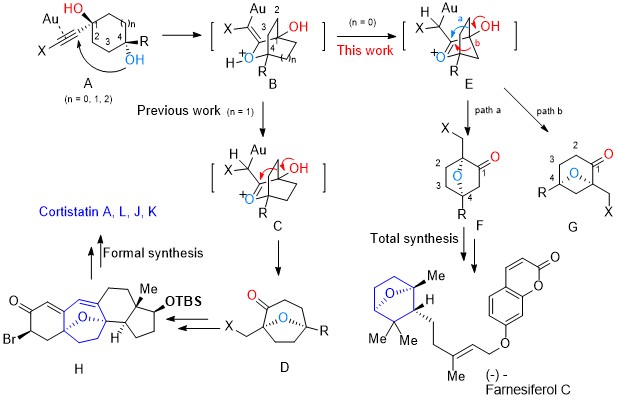
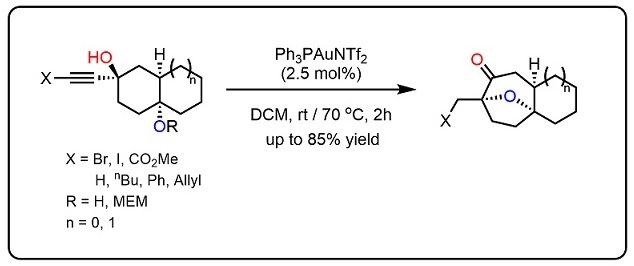
101.Regio- and Stereoselective Syntheses of 7-Oxabicyclo[2.2.1]heptanes via a Gold(I)-Catalyzed Cycloisomerization of Alkynediols: Asymmetric Total Synthesis of Farnesiferol C
Yue-Qing Gu, Peng-Peng Zhang, Jun-Kai Fu, Song Liu, Yu Lan,* Jian-Xian Gong,* and Zhen Yang*
Adv. Synth. Catal. 2016, 358, 1392-1397
A highly regio- and stereoselective method to construct a broad range of 7-oxabicyclo[2.2.1]heptanes, which proceeds through a sequential reaction involving gold(I)-catalyzed cycloisomerization of alkynediols and sequential semi-pinacol-type 1,2-alkyl migration, was developed. The developed chemistry was applied to the asymmetric total synthesis of the natural product farnesiferol C.
100.Gold-Catalyzed Enantio- and Diastereoselective Syntheses of Left Fragments of Azadirachtin/Meliacarpin-Type Limonoids
Hang Shi, Ceheng Tan, Weibin Zhang, Zichun Zhang, Rong Long, Jianxian Gong, Tuoping Luo*, and Zhen Yang*
99.Towards a general diastereoselective route to oxabicyclo[3.2.1]octanes via a gold-catalysed cascade reaction
Junkai Fu, Yueqing Gu, Hao Yuan, Tuoping Luo, Song Liu, Yu Lan*, Jianxian Gong*, Zhen Yang*
Nat. Commun., 2015, 6:8617. DOI: 10.1038/ncomms9617 (2015)
The development of an efficient diastereoselective synthesis of the oxabicyclo[3.2.1]octane ring system bearing two oxygenated quaternary chiral centres represents a significant challenge. This motif can be found in a wide range of natural products with significant biological activities. Here we report the synthesis of such kind of scaffold using a cyclohexane-trans-1,4-diol with an alkyne side chain in the presence of Au(I) catalyst. This is a domino process in which two C–H, two C–O and one C–C bond is assembled through a sequence of cyclization/semi-pinacol rearrangements. This strategy has been successfully applied to the asymmetric formal total synthesis of (+)-cortistatins.
98.Direct construction of vicinal all-carbon quaternary stereocenters in natural product synthesis
Rong Long, Jun Huang, Jianxian Gong* and Zhen Yang*
Nat. Prod. Rep., 2015,32, 1584
Molecules containing vicinal all-carbon quaternary stereocenters are found in many secondary metabolites, and they exhibit a variety of biological and pharmacological activities. However, the construction of such a structural motif remains a significant challenge in natural product synthesis. Only in recent years have considerable efforts been made to construct vicinal quaternary stereocenters in a single-step operation. In this review, we focus on the different types of methods that have been successfully used in the total synthesis of natural products. Based on the classified reactions for the simultaneous generation of vicinal all-carbon quaternary stereocenters, the total syntheses of the natural products are discussed, placing emphasis on the diastereoselective preparation of vicinal quaternary carbon centers and the subsequent total syntheses.