Publications
80.Strategic innovation in the total synthesis of complex natural products using gold catalysis
Yun Zhang, Tuoping Luo* and Zhen Yang*
Nat. Prod. Rep., 2014, 31, 489
Novel organic reactions drive the advance of chemical synthesis in the same way that enabling technologies drive new scientific discoveries. One area of organic methodology that has undergone significant growth during the last decade is that of homogeneous gold-catalyzed transformations. This trend has been further enhanced by the employment of gold catalysis on a routine basis to accomplish the total synthesis of natural products. In particular, the superior π acidity of the cationic gold complex for the activation of alkynes and allenes towards nucleophilic addition has significantly enriched the toolkit of transformations available to the total synthesis community, and inspired a new era of creativity in terms of the strategic disconnection of target compounds during their retrosynthetic analysis. Instead of simply supplementing the many existing reviews of gold catalysis, this review has been organized from the perspective of synthetic target families, with particular emphasis on the use of gold-catalyzed transformations during the late stages of syntheses involving complicated substrates, and cascade reactions that significantly increase molecular complexity.
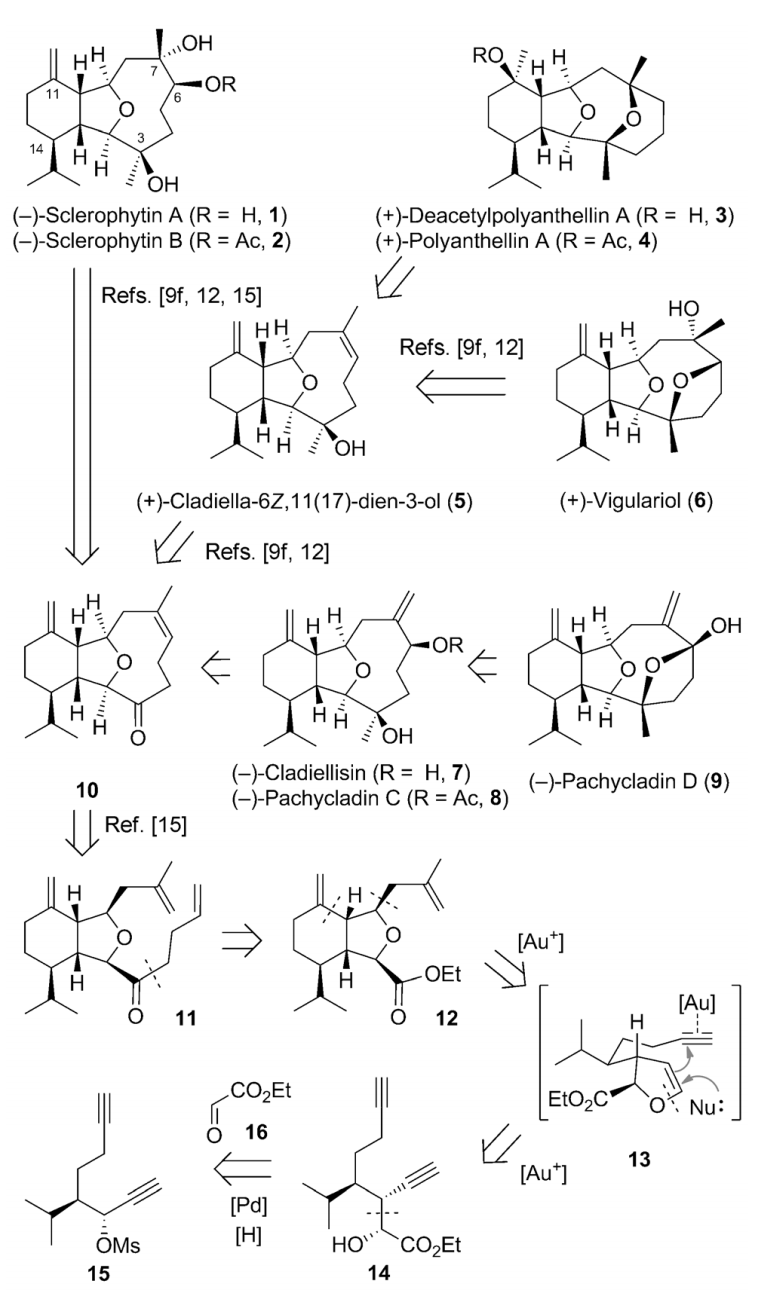
79.Collective Synthesis of Cladiellins Based on the Gold-Catalyzed Cascade Reaction of 1,7-Diynes
Guozong Yue, Yun Zhang, Lichao Fang, Chuang-chuang Li, Tuoping Luo,* and Zhen Yang*
Angew. Chem. Int. Ed. 2014, 53, 1837
The cladiellin family of natural products, which includes molecules with various biological activities, continues to invite new synthetic studies. A gold-catalyzed tandem reaction of 1,7-diynes to construct the 6-5-bicyclic ring systems that are present in a number of natural products was developed. This reaction was applied as the key step to realize the formal and total syntheses of nine members of the cladiellin family in an enantio- and diastereoselective manner. This modular and efficient approach could also be used for the construction of other cladiellins, as well as their analogues, for follow-up studies.
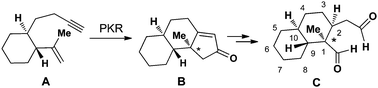
78.Development of an expedient intramolecular Pauson–Khand reaction approach to stereoselectively construct the trans-decalin with a C1 quaternary chiral center
Li-Li Shi, Hong-Juan Shen, Li-Chao Fang, Jun Huang, Chuang-Chuang Li * and Zhen Yang *
Stereoselective synthesis of the trans-decalin subunit with a defined C1 quaternary chiral center has been achieved by the Pauson–Khand reaction (PKR) as a key step. The developed chemistry offers an alternative to the IMDA reaction that has been used for the syntheses of trans-decalin based biologically active natural products.
77. Asymmetric Total Synthesis of (+)-Fusarisetin A via the Intramolecular Pauson–Khand Reaction
Jun Huang, Lichao Fang, Rong Long, Li-Li Shi, Hong-Juan Shen, Chuang-chuang Li *, and Zhen Yang *
• Top10 Most Accessed Article in 07/2013.
• Highlighted in Synfacts, 2014, 10, 8.
An asymmetic total synthesis of (+)-fusarisetin A has been achieved. The essential to our strategy was the application of the intramolecular Pauson–Khand reaction for the stereoselective construction of the trans-decalin subunit of (+)-fusarisetin A with a unique C16 quarternary chiral center. The developed chemistry offers an alternative to the IMDA reaction that has been used for fusarisetin A, and is applicable to analogue synthesis for biological evaluation.

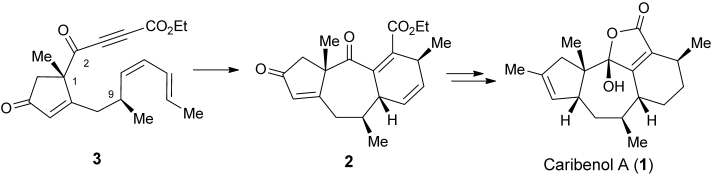
76.Asymmetric, Protecting-Group-Free Total Synthesis of (+)-Caribenol A
Jing-Chun Han, Dr. Lian-Zhu Liu, Prof. Dr. Chuang-Chuang Li,*, Prof. Dr. Zhen Yang,*
• Highlighted in Synfacts, 2013, 9(8)3, 809
Caribenol Queen: A new asymmetric, protecting-group-free synthesis of the marine tetracyclic diterpenoid (+)-caribenol A (1) has been achieved. The enantioselective synthesis employed (S)-methyl 1-methyl-2-oxocyclopent-3-enecarboxylate as a chiral scaffold, and an intramolecular Diels–Alder (IMDA) reaction of substrate 3 afforded the [5.7.6] tricyclic core in compound 2.