Publications
57.A Concise Approach for the Total Synthesis of Pseudolaric Acid A
T. Xu, C. C. Li*, Z. Yang*
• “Highlight syntheses” in Annu. Rep. Prog. Chem., Sect. B: Org. Chem., 2012.
A new strategy for the stereoselective total synthesis of natural product pseudolaric acid A (1) was accomplished in 16 steps from commercially available starting material, featuring a samarium diiodide (SmI2)-mediated intramolecular alkene-ketyl radical cyclization and a ring-closing metathesis (RCM) reaction to stereoselectively cast the unusual trans-fused [5–7]-bicyclic core of pseudolaric acid A (1).

56.Asymmetric Total Synthesis and Structural Elucidation of NFAT-68
L. Wang, Y. M. Xi, S. L. Yang, R. Zhu, Y. F. Liang, J. H. Chen*, Z. Yang*
Total synthesis of NFAT-68 (7) has been achieved and its relative stereochemistry has been determined. A key step thereof is the utilization of the chelation-controlled vinylogous Mukaiyama aldol reaction (VMAR) to stereoselectively synthesize the syn-aldol product 8. This developed chemistry is anticipated to have wider application in total syntheses of many other natural products.

55.Total Synthesis of Drimane-Type Sesquiterpenoids Enabled by a Gold-Catalyzed Tandem Reaction
H. Shi, L. C. Fang, C. H. Tan, L. L. SHi, W. B. Zhang, C. C. Li*, T. P. Luo*, Z. Yang*
J. Am. Chem. Soc. 2011, 132, 14944
• Highlighted in Org. Chem. Highlights, August, 2012.
Development of a gold-catalyzed tandem reaction of 1,7-diynes with both internal and external nucleophiles was realized, which constructed five chemical bonds, two rings, and two stereogenic centers in a single step. Based on the novel cascade transformation, we achieved a unified strategy toward the stereoselective total syntheses of C-15 oxygenated drimane-type sesquiterpenoids and their analogues, which provided the natural products kuehneromycin A, antrocin, anhydromarasmone, and marasmene as a proof-of-concept study.

*This publication was highlighted on http://www.organic-chemistry.org/Highlights/2012/13August.shtm
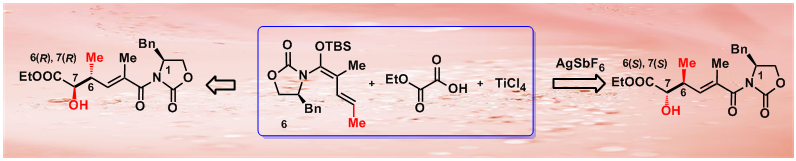
54.An Unprecedented Silver Salt Effect Switches the Facial Selectivity in the Vinylogous Mukaiyama Aldol Reaction
Y. Liang, L. Wang, R. Zhu, L. J. Deng, Y. Yang, J. M. Quan, J. H. Chen*, Z. Yang*
Adv. Synth. Catal. 2010, 352, 2387.
Silver hexafluroantimonate was identified as a highly efficient agent to reverse the facial selectivity of the titanium chloride-mediated vinylogous Mukaiyama aldol reaction (VMAR), and this unprecedented reaction provides a concise, diastereoselective synthesis of δ-hydroxy-α,γ-dimethyl-α,β-unsaturated carbonyl units from readily available chiral vinylketene silyl N,O-acetal 6 and ethyl glyoxylate 8.
53.Organopalladium(IV) chemistry
L. M. Xu, B. J. Li, Z. Yang, Z. J. Shi*
Chem. Soc. Rev. 2010, 39, 712.
Although the chemistry of Pd(0), Pd(I) and Pd(II) is well established, high oxidation state Pd(IV)complexes are less well-known. This situation has highly changed in recent years. Many well-defined Pd(IV) complexes has been isolated and characterized, providing evidence for a series of proposed Pd(II)/Pd(IV) catalytic reactions. A deep understanding of the behavior of Pd(IV)complexes could lead to the design and development of novel reactions that could not be accessed by traditional Pd(0)/Pd(II) chemistry. This critical review describes the stoichiometric reactions of Pd(IV) complexes and discusses their potential mechanism in catalytic reactions (137 references).