Publications
65.Diastereoselective Total Synthesis of (±)-Schindilactone A, Part 2: Construction of the Fully Functionalized CDEFGH Ring System
Yong Li, Zhi-Xing Chen, Dr. Qing Xiao, Qin-Da Ye, Tian-Wen Sun, Fan-Ke Meng, Dr. Wei-Wu Ren, Lin You, Ling-Min Xu, Yue-Fan Wang, Dr. Jia-Hua Chen,*, Dr. Zhen Yang,*
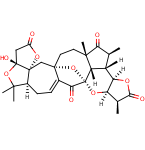
The successful synthesis of the highly complex model compound (2) of the CEFGH ring system of schindilactone A (1) is described. Several synthetic methodologies were developed and applied to achieve this goal, including ring-closing metathesis (RCM) and Co–thiourea-catalyzed Pauson–Khand reactions. Furthermore, two independent approaches were developed for the construction of the GH ring of model compound 2, the key steps of which included Pd–thiourea-catalyzed carbonylative annulation, methylation, and sequential RCM/oxa-Michael-addition reactions. The chemistry developed herein has provided a greater understanding of the synthesis of schindilactone A (1) and its analogous compounds of the same family.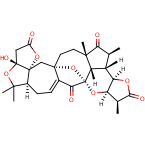
64.Diastereoselective Total Synthesis of (±)-Schindilactone A, Part 3: The Final Phase and Completion
Dr. Wei-Wu Ren, Zhi-Xing Chen, Dr. Qing Xiao, Yong Li, Tian-Wen Sun, Zi-Yang Zhang, Qin-Da Ye, Fan-Ke Meng, Lin You, Ming-Zhe Zhao, Ling-Min Xu, Prof. Dr. Ye-Feng Tang,*, Prof. Dr. Jia-Hua Chen,*, Prof. Dr. Zhen Yang,*
The final phase for the total synthesis of (±)-schindilactone A (1) is described herein. Two independent synthetic approaches were developed that featured Pd–thiourea-catalyzed cascade carbonylative annulation reactions to construct intermediate 3 and a RCM reaction to make intermediate 4. Other important steps that enabled the completion of the synthesis included: 1) A Ag-mediated ring-expansion reaction to form vinyl bromide 17 from dibromocyclopropane 30; 2) a Pd-catalyzed coupling reaction of vinyl bromide 17 with a copper enolate to synthesize ketoester 16; 3) a RCM reaction to generate oxabicyclononenol 10 from diene 11; 4) a cyclopentenone fragment in substrate 8 was constructed through a Co–thiourea-catalyzed Pauson–Khand reaction (PKR); 5) a Dieckmann-type condensation to successfully form the A ring of schindilactone A (1). The chemistry developed for the total synthesis of schindilactone A (1) will shed light on the synthesis of other family members of schindilactone A.
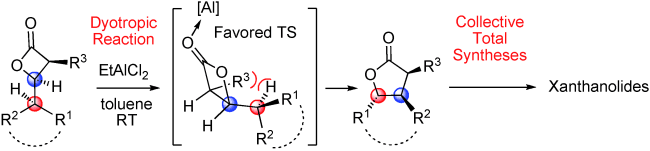
63.Enantioselective and Collective Syntheses of Xanthanolides Involving a Controllable Dyotropic Rearrangement of cis-b -Lactones
W. W. Ren, Y. C. Bian, Z. Y. Zhang, H. Shang, P. T. Zhang, Y. J. Chen, Z. Yang*, T. P. Luo*, and Y. F. Tang*
Angew. Chem. Int. Ed. 2012, 51, 6984
Let’s swap: A scalable, atom-economic, enantio-, and diastereoselective synthetic route to trisubstituted γ-butyrolactones based on a Wagner–Meerwein-type dyotropic rearrangement of cis-β-lactones is described (see scheme). This methodology was applied in efficient and protecting-group-free formal syntheses and total syntheses of various xanthanolide natural products.
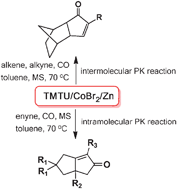
62.CoBr2-TMTU-Zinc Catalysed-Pauson-Khand Reaction
Y. F. Wang, L. M. Xu, R. C. Yu, J. H. Chen*, Z. Yang*
A cobalt–TMTU complex, derived from the in situ reduction of CoBr2 with Zn in the presence of TMTU, can catalyze Pauson–Khand reaction at a balloon pressure of CO, which enables the synthesis of structurally diverse cyclopentenones. This catalytic system works efficiently for both intermolecular and intramolecular PK reactions.
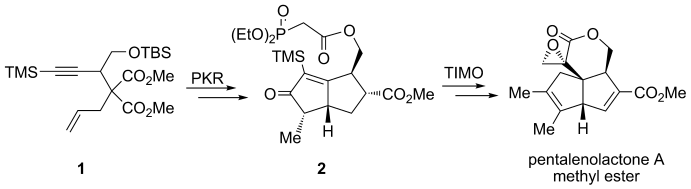
61.Total Synthesis of (±)-Pentalenolactone A Methyl Ester
Q. Liu, G. Z. Yue, N. Wu, G. Lin, Y. Z . Li, J. M. Quan, C. C. Li*, G. X. Wang*, Z. Yang*
Angew. Chem. Int. Ed. 2012, 51, 48, 12072
• Top 10 Most Accessed Article in 10/2012.
• Highlighted in Chin. J. Org. Chem., January, 2013.
• Highlighted in Chemistry World, November, 2012.
Ringing it up: The methyl ester of pentalenolactone A has been obtained through the stereoselective synthesis of a cyclopentenone by a combination of the Co-mediated Pauson–Khand reaction (PKR) of enyne 1, and the construction of a quaternary-carbon-based strained α-methylidene-δ-pentyrolactone core through a trimethylsilyl (TMS) mediated, telescoped intramolecular Michael olefination (TIMO) reaction of keto-phosphonate 2.