Publications
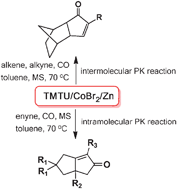
62.CoBr2-TMTU-Zinc Catalysed-Pauson-Khand Reaction
Y. F. Wang, L. M. Xu, R. C. Yu, J. H. Chen*, Z. Yang*
A cobalt–TMTU complex, derived from the in situ reduction of CoBr2 with Zn in the presence of TMTU, can catalyze Pauson–Khand reaction at a balloon pressure of CO, which enables the synthesis of structurally diverse cyclopentenones. This catalytic system works efficiently for both intermolecular and intramolecular PK reactions.
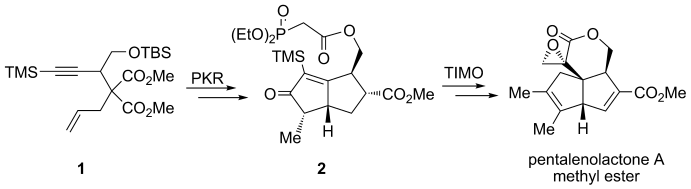
61.Total Synthesis of (±)-Pentalenolactone A Methyl Ester
Q. Liu, G. Z. Yue, N. Wu, G. Lin, Y. Z . Li, J. M. Quan, C. C. Li*, G. X. Wang*, Z. Yang*
Angew. Chem. Int. Ed. 2012, 51, 48, 12072
• Top 10 Most Accessed Article in 10/2012.
• Highlighted in Chin. J. Org. Chem., January, 2013.
• Highlighted in Chemistry World, November, 2012.
Ringing it up: The methyl ester of pentalenolactone A has been obtained through the stereoselective synthesis of a cyclopentenone by a combination of the Co-mediated Pauson–Khand reaction (PKR) of enyne 1, and the construction of a quaternary-carbon-based strained α-methylidene-δ-pentyrolactone core through a trimethylsilyl (TMS) mediated, telescoped intramolecular Michael olefination (TIMO) reaction of keto-phosphonate 2.
60.Reverse Regioselectivity in the Palladium(II) Thiourea Catalyzed Intermolecular Pauson–Khand Reaction
N. Wu, L. J. Deng, L. Z. Liu, Q. Liu, C. C. Li*, Z. Yang*
Which way? Palladium thiourea catalyzed Pauson–Khand reaction of norbornene with substituted alkynoates to afford selectively either α-carboxylated adducts or β-carboxylated adducts with the reverse regioselectivity has been demonstrated for the first time. Control of the regioselectivity in this transformation is governed by the addition of LiCl. A mechanistic interpretation to account for this observed regioselectivity is presented.
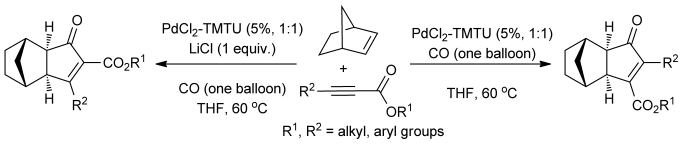
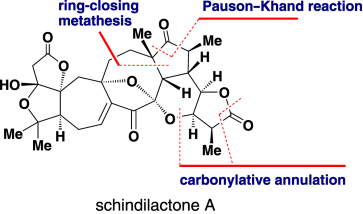
59.Diastereoselective Total Synthesis of (±)-Schindilactone A
Q. Xiao, W. W. Ren, Z. X. Chen, T. W. Sun, Y. Li, Q. D. Ye, J. X. Gong, F. K. Meng, L. You, Y. F. Liu, M. Z. Zhao, L. M. Xu, Z. H. Shan, Y. Shi,Y. F. Tang*, J. H. Chen*, Z. Yang*
Angew. Chem. Int. Ed. 2011, 50, 7373.
All together: A concise strategy for the first diastereoselective total synthesis of (±)-schindilactone A is reported. The synthesis features a ring-closing metathesis, a thiourea/cobalt-catalyzed Pauson–Khand reaction, and a thiourea/palladium-catalyzed carbonylative annulation reaction. The chemistry can be applied to the synthesis of structures related to schindilactone A.
58.Formal Synthesis of Cortistatins
L. C. Fang, Y. Chen, J. Huang, L. Z. Liu, J. M. Quan, C. C. Li*, Z. Yang*
A unified strategy toward the asymmetric facile construction of the [6.7.6.5]oxapentacyclic skeleton of cortistatins is reported, featuring intramolecular Diels−Alder (IMDA), oxidative dearomatization, and an oxy-Michael addition reaction.





