Publications
162.Palladium-Catalyzed Intramolecular Diarylation of 1,3-Diketone in Total Synthesis of (±)-Spiroaxillarone A
Tingting Cao, Lei Zhu,* Jun Huang,* and Zhen Yang*
Org. Lett. 2022, 24,(7), 1491–1495
A sterically congested all-carbon quaternary center was installed for the first time via a Pd-catalyzed cascade diarylation with aryl bromides and acyclic 1,3-diketones. This method was used as a key step in the total synthesis of (±)-spiroaxillarone A. Computational experimental results indicated that the selective diarylation is accelerated by the higher free-energy barriers of the endothermic transmetalation and reductive elimination in the first arylation step.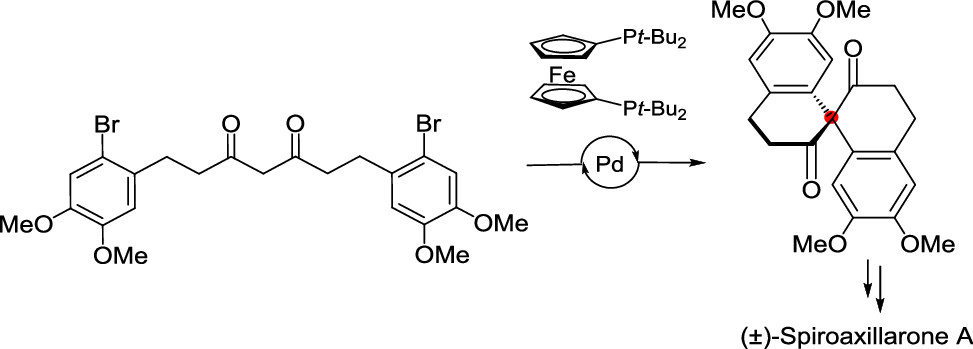
161.Concise Synthesis of 7-Deoxypsammaplysins K and O and 7-Deoxyceratinamide A by 1,3-Dipole Cycloaddition
Lijie Zhang, Rongya Wang, Chao Wang, Bingyan Liu, Jinfeng Yang, Zhongchao Zhang, Jun Huang,*and Zhen Yang*
Org. Lett. 2022, 24(21), 3786–3791
A spiro-oxepin isoxazoline skeleton was constructed via 1,3-dipole cycloaddition as a key step, which enabled the total syntheses of 7-deoxyceratinamide A and 7-deoxypsammaplysins K and O. The developed chemistry could be applied to total synthesis of structurally diverse spiro-oxepin isoxazoline-based marine natural products.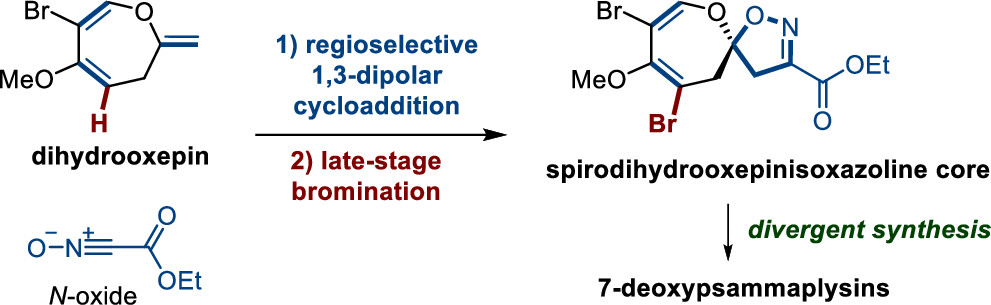
160.Total Syntheses of Vicinal Dichloride Monoterpenes Enabled by AzaBellus−̌ Claisen Rearrangement
Jiangqun Cheng, Yuan-He Li, Jun Huang,* and Zhen Yang*
Org. Lett. 2021, 23(21), 8465–8470
Diastereoselective syntheses of syn– and anti-vicinal dihalides were achieved via an aza-Belluš–Claisen rearrangement, which involved the reaction of an α-chloro carboxylic acid chloride with halogen-substituted trans-allyl morpholines in the presence of Lewis acids. The developed method was used for the total synthesis of a group of monoterpene natural products bearing vicinal dichloride subunits.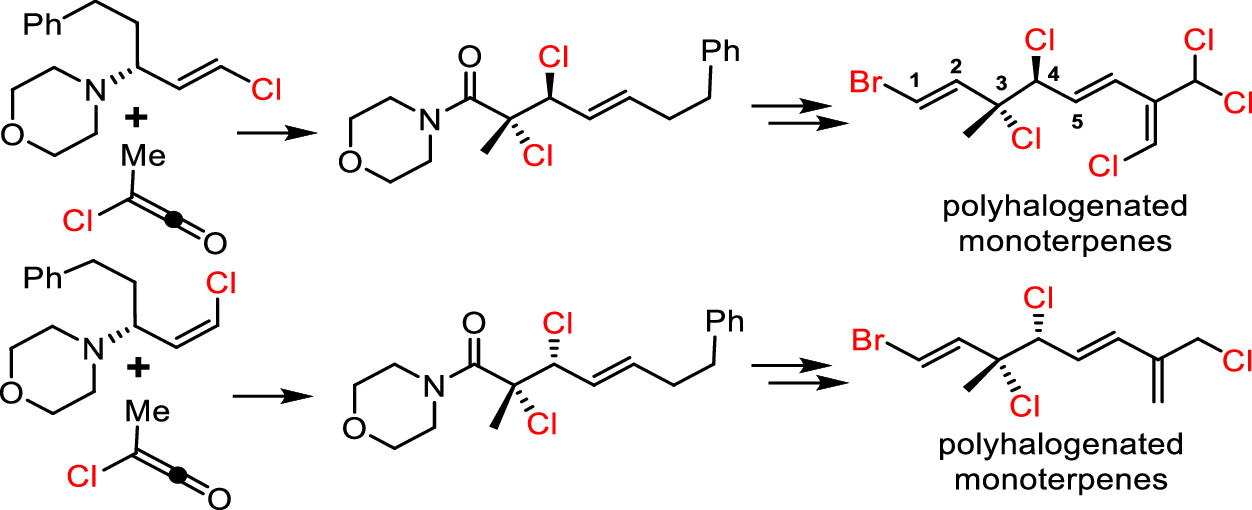
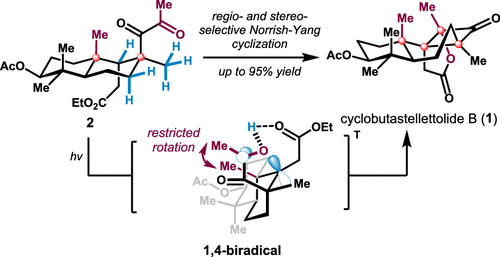
159.Total Synthesis of (+)-Cyclobutastellettolide B
Zhongchao Zhang, Sijia Chen, Fu Tang, Kai Guo, Xin-Ting Liang, Jun Huang,* and Zhen Yang*
J. Am. Chem. Soc. 2021, 143,(43), 18287–18293
A convenient enantioselective total synthesis of (+)-cyclobutastellettolide B via a strategy that involves a diastereoselective Johnson–Claisen rearrangement, a regioselective cyclopropoxytrimethylsilane ring-opening reaction, and a Norrish–Yang cyclization is described. The results of computational and experimental studies indicate that the regio- and stereoselectivity of the Norrish–Yang reaction are controlled by the C–H bond dissociation energy and restricted rotation of the C13–C14 bond.
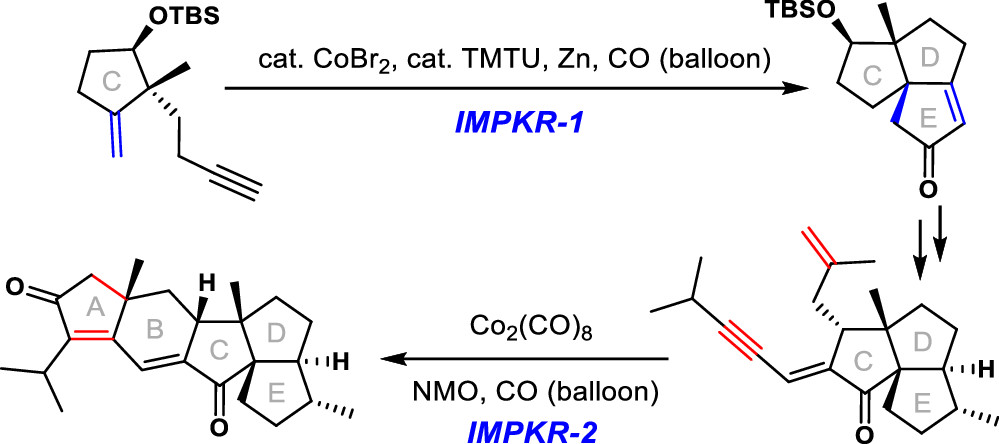
158.A Synthetic Route to The Core Structure of (−)-Retigeranic Acid A
Xiao Wang, Dian Li, Junlin Zhang, Jianxian Gong, Junkai Fu,* and Zhen Yang*
Org. Lett. 2021, 23,(13), 5092–5097
Retigeranic acid A is a uniquely structured pentacyclic sesterterpene bearing eight stereogenic centers. We report a concise route to the core structure of (−)-retigeranic acid A. The stereochemistry of its six chiral centers and three quaternary carbon centers was well-controlled. This route features two intramolecular Pauson–Khand reactions (IMPKRs): the first forged the D and E rings to deliver the triquinane subunit, and the second constructed the A and B rings and diastereoselectively installed the quaternary C6a center.