Publications
135.Concise synthesis of the core structure of madreporanone by Rh-catalyzed [3+2] cycloaddition
Rong Long, Zhen Yang*
Tetrahedron 2019, 75 (12), 1746-1750
A model study toward total synthesis of madreporanone, a novel diterpene isolated from Azorella madreporica, was investigated. The [3.3.0] bicyclic core of madreporanone bearing a cis-configured isopropyl group and two vicinal quaternary carbon centers was stereoselectively constructed via an intramolecular Rh-catalyzed [3 + 2] cycloaddition in a single step.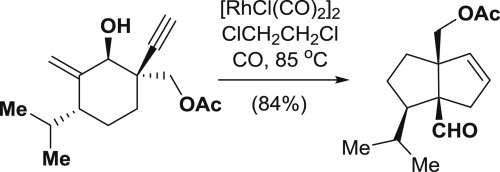
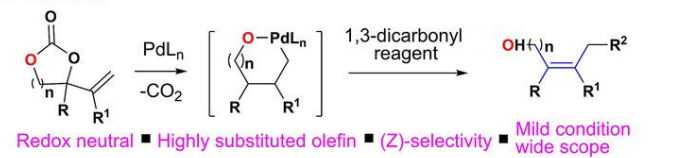
134.Pd-Catalyzed Decarboxylative Allylation for Stereoselective Syntheses of Allylic Alcohols bearing a Quaternary Carbon Center
Linlin Shi, Yingdong He, Jianxian Gong*, Zhen Yang*
Asian J. Org. Chem 2019, 8 (6), 823-827
Olefins play a vital role in the fields of bioscience, medicine, and chemistry. Stereo‐defined olefins are important resource in both chemical laboratory and industry. Using a palladium‐catalyzed decarboxylative transformation of vinyl cyclic carbonates, we have developed an efficient and reliable method for the direct construction of highly substituted olefins with the concomitant formation of a new Csp3‐Csp3 bond. The desired olefins could be generated in reasonable yields with good stereoselectivities, in contrast to the existing methods, which are usually non‐stereoselective. The palladium‐catalyzed decarboxylative transformation will undoubtedly provide new synthetic routes for the formation of multi‐substituted allylic scaffolds.
133.Total syntheses of dehydrobotrydienal, dehydrobotrydienol and 10-oxodehydrodihydrobotrydial
Zichun Zhang, Dandan Zhao, Yingdong He, Zhen Yang, Jianxian Gong
Chin. Chem. Lett. 2019, 30 (8), 1503-1505
In this paper, we report the concise total syntheses of three botryane sesquiterpenoids: dehydrobotrydienal, dehydrobotrydienol, and 10-oxodehydrodihydrobotrydial. The key transformations include tandem Co-tetramethylthiourea-catalyzed Pauson–Khand and 6π-electrocyclization reactions to forge the tricyclic core structure of the botryanes, and further oxidative aromatization and oxidation to complete the total syntheses.
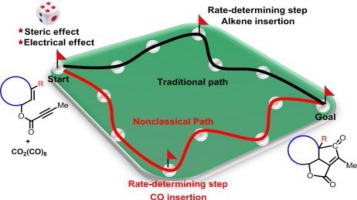
132.Theoretical prediction on the reactivity of the Co-mediated intramolecular Pauson-Khand reaction for constructing bicyclo-skeletons in natural products
LeiZhu, ZheyuanWang, SongLiu, TaoZhang, ZhenYang, RuopengBai, YuLan
Chin. Chem. Lett. 2019, 30 (4), 889-894
The Co2(CO)8-mediated intramolecular Pauson-Khand reaction is an efficient approach for constructing polycyclic skeletons. Recently, some of us reported a series of this type reactions involving sterically-hindered enynes for synthesizing natural products with reasonable reaction rates and yields. However, the reason for the high reactivity of the reaction remains unclear. We employed density functional theory calculations to clarify the mechanism and reactivity for this reaction. In contrast with chain olefin reactants, CO insertion is considered to be the rate-determining step for the overall Pauson-Khand reaction of cyclooctene derivatives. The reduced activation free energy for the alkene insertion step is attributed to: i) the electron-withdrawing group in close proximity to the CC triple bond enhancing the reactivity of the alkyne moiety; ii) lower steric hindrance during alkene insertion when using the cyclooctene derivative. The effect of the substituent on the Co2(CO)8-mediated intramolecular Pauson-Khand reaction was then investigated. Internal alkenes exhibit lower reactivity than terminal alkenes because of the steric hindrance introduced by the substituted group. The cis internal alkene exhibits higher reactivity than the trans internal alkene. An ester group in close proximity to the CC triple bond significantly enhances the reactivity.
131.Stereoselective Pd-Catalyzed Decarboxylative Allylation: Assembly of Highly Functionalized Allylic Amines Bearing a Quaternary Center
Linlin Shi, Yingdong He, Yuanyuan Chang, Nan Zheng, Zhen Yang*, and Jianxian Gong*
Org. Lett. 2019, 21(9), 3077-3080
Here, we report a practical and reliable methodology to direct construction of tri- and tetrasubstituted olefins bearing an allylic amine, with the concomitant construction of the sterically congested quaternary stereocenter through stereoselective palladium-catalyzed cascade decarboxylation of vinyloxazolidinones.